A kind of recombinant protein VO and its preparation method and application
A technology of recombinant protein and Escherichia coli, applied in chemical instruments and methods, recombinant DNA technology, pharmaceutical formulations, etc., can solve the problem of preparing antibodies without OmpA protein, achieve good immune protection effect, maintain spatial conformation and immunogenicity, The effect of mild purification conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
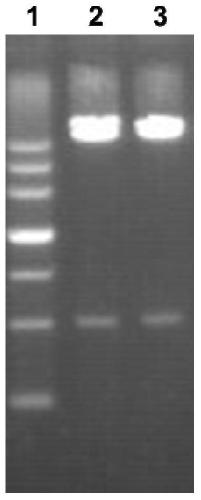
[0039] Example 1 Gene synthesis and subcloning
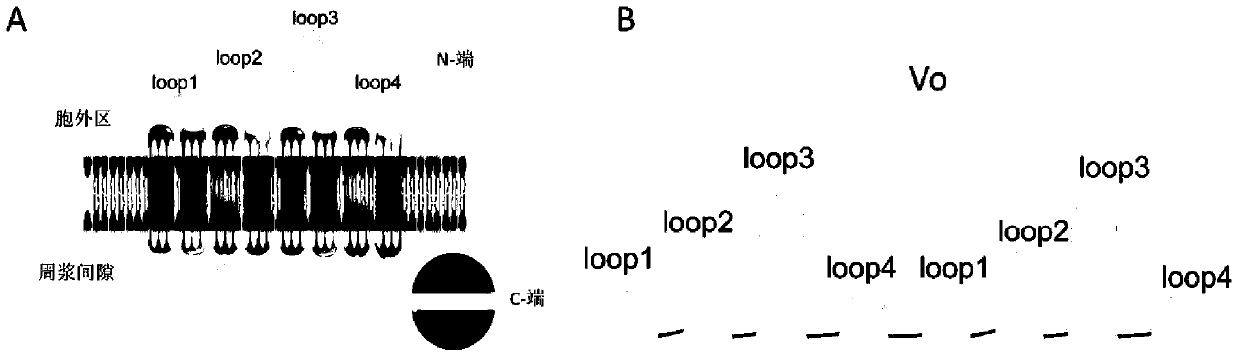
[0040] 1. According to figure 1 Based on the design idea, the recombinant protein Vo containing Loop1, Loop2, Loop3, Loop4 and protein linker was designed. The synthesis of DNA encoding Vo and the connection of sequence and pGEX-6p-2 were synthesized by Shanghai Sangong Bioengineering Co., Ltd.
[0041] 2. Transformation of recombinant plasmids
[0042] Take 3 tubes of Escherichia coli XL1blue competent cells (Shanghai Chaoyan Biotechnology Co., Ltd.) from the -80°C refrigerator, and add pGEX-6P-2 plasmid (GE Healthcare Life Sciences) to the first tube as a positive control; add 1ulVo to the second tube Synthetic plasmid; no exogenous DNA was added to the third tube as a negative control. Ice bath for 50min, heat shock in 42℃ metal bath for 90s, rapid ice bath for 2min. Add 600 μl LB blank medium, mix well, and shake at 220rp for 1 hour in a shaker at 37°C.
[0043] Each tube was centrifuged at 5000 rpm for 3 min at room t...
Embodiment 2
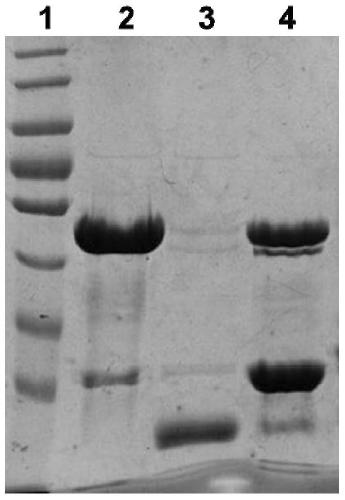
[0049] Example 2 Induced expression, purification and identification of expression form of recombinant fusion protein Vo in prokaryotic expression system-Escherichia coli
[0050] 1. Vo-induced expression
[0051] 1) Take 100 μL of the overnight cultured pGEX-6P-2-Vo / XL-1blue bacterial solution and add it to 10 mL of Amp+ resistant LB medium, culture overnight at 180 rpm at 37°C, take 400 μL of the overnight cultured bacterial solution and add 20 mL of Amp+ resistant LB medium (the rest of the bacterial solution was stored in a refrigerator at 4°C for later use), cultured at 37°C for 2-3 hours at a rotational speed of 200 rpm, and when the secondary activation reached OD600 of 0.8-1.0, 4 μL of IPTG was added to make the final concentration 200 μM. Then place on a shaking table to induce expression at 30°C for 3h.
[0052] 2) Take out the bacterial solution after induced expression, centrifuge at 12,000rpm for 5min, discard the supernatant, add 1mL lysisbuffer (20mM PB, pH 7...
Embodiment 3
[0059] Example 3 Preparation of Vo antigen
[0060] 1. Amplify culture to obtain protein
[0061] Take 400 μL of the spare pGEX-6P-2-Vo / XL-1blue bacterial solution stored in a 4°C refrigerator and add it to 20 mL of LB medium containing Amp resistance for one activation. Add the primary activated bacterial solution to 400mL LB medium containing Amp resistance for secondary activation, culture at 37°C for 3-4h until the OD600 is 1.0, add 80μLPTG (final concentration is 200μM) and place in a shaker at 16°C overnight After induction, 12000rpm was centrifuged for 15min to collect the thalline, and after adding 20mL lysis buffer (same as Example 2) to resuspend the thallus, the bacterium liquid was subjected to ultrasonic lysis for 3min (200V), and the supernatant and 800 μL of GST fusion protein were collected. Glutathione Sepharose 4B (GE Company) gel beads (beads) binding treatment; then SDS-PAGE gel electrophoresis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com