A kind of method that transfer hydrogenation method prepares aminonaphthol sulfonic acid
A technology for aminonaphtholsulfonic acid and nitronaphtholsulfonic acid is applied in the field of preparing aminonaphtholsulfonic acid by transfer hydrogenation, which can solve the problems of corrosion equipment process operation, harsh preparation conditions, pollution of the environment and the like, and avoid pollution problems. , The effect of good product quality and easy process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] First add 25.3g (0.1mol) 1-naphthol-2-nitroso-5-sulfonic acid in the reaction vessel, then add 50% of 1-naphthol-2-nitroso-5-sulfonic acid weight Ethanol, add 80% hydrazine hydrate 9.0g (0.144mol), then add 5% Raney nickel catalyst of 1-naphthol-2-nitroso-5-sulfonic acid weight, stir at room temperature, control the reaction temperature 30 ℃ , Stirring and reacting for 3 hours, the conversion rate of raw materials reaches above 98.0%. The catalyst was filtered out at room temperature to obtain a solution of the product, which was concentrated and acidified to obtain the product.
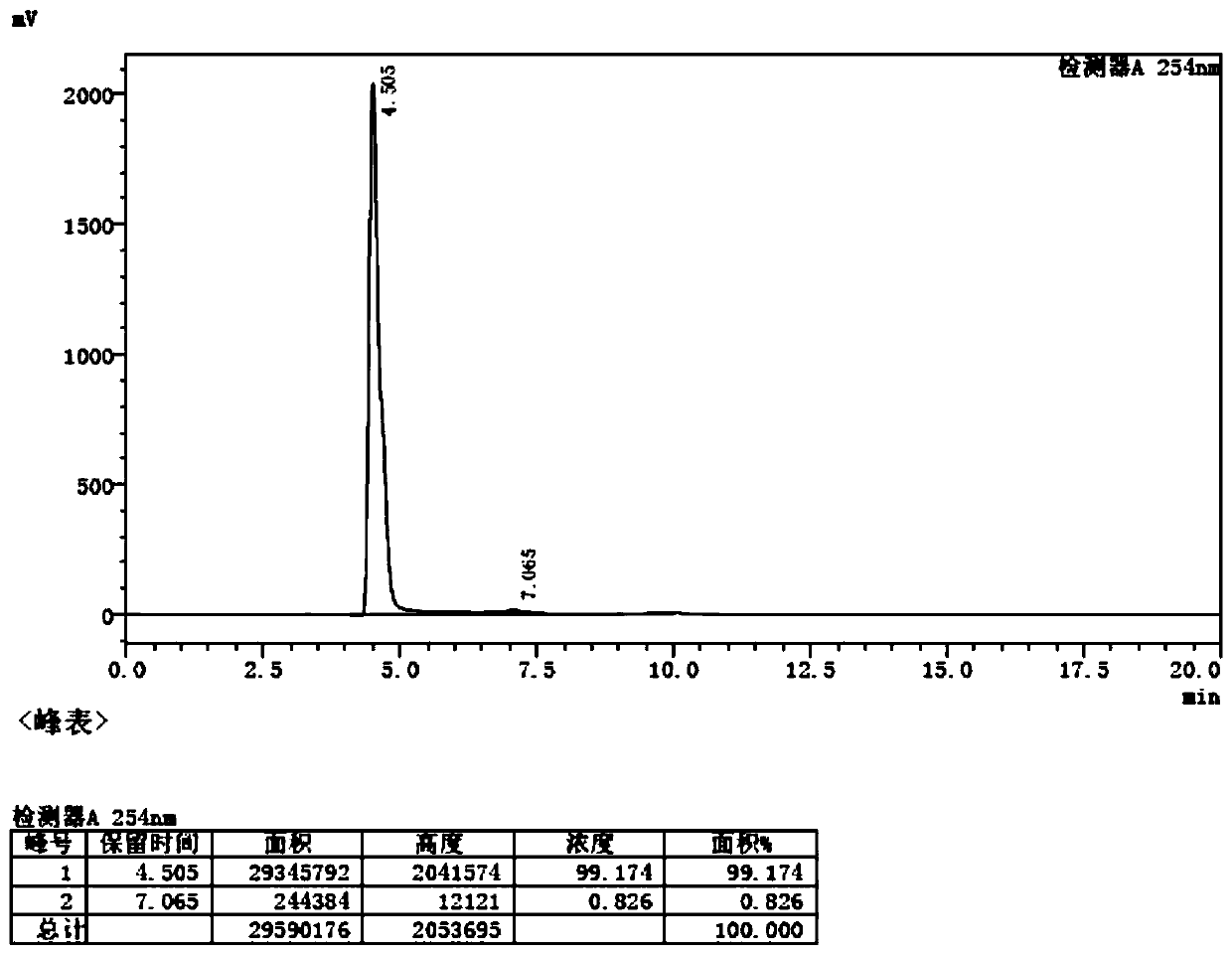
[0046] figure 1 It is the HPLC collection of illustrative plates of the product of this embodiment, and the HPLC detection condition is: instrument parameter: Agilent high performance liquid chromatograph 1100, diode array detector, chromatographic column: C18 46 * 250mm, 5 μ m, mobile phase: V methanol: V water: V acetic acid :V triethylamine=14:85.1:0.6:0.3, flow rate: 1mL / min, detection w...
Embodiment 2
[0049] First add 25.3g (0.1mol) 1-naphthol-2-nitroso-5-sulfonic acid in the autoclave, add 9.0g (0.144mol) of 80% hydrazine hydrate, then add 1-naphthol-2-sulfonic acid 5 times the ethanol of nitro-5-sulfonic acid weight, then add 5% Pt / C catalyst of 1-naphthol-2-nitroso-5-sulfonic acid weight, stir evenly at room temperature, control reaction temperature 20 ℃ , Stirring and reacting for 3 hours, the conversion rate of raw materials reached above 98.2%. The catalyst was filtered out at room temperature to obtain a solution of the product, which was concentrated and acidified to obtain the product.
[0050] Liquid phase analysis showed that the product content was 98.6%, and the yield was 85%.
Embodiment 3
[0052] First add 25.3g (0.1mol) 1-naphthol-2-nitroso-5-sulfonic acid in the autoclave, add 6.26g (0.1mol) of 80% hydrazine hydrate, then add 1-naphthol-2-nitroso nitro-5-sulfonic acid 2 times the weight of ethanol, then add 1-naphthol-2-nitroso-5-sulfonic acid 5% Pd / C catalyst, stir evenly at room temperature, control the reaction temperature 40 ℃ , Stirring and reacting for 4 hours, the conversion rate of raw materials reached above 98.4%. The catalyst was filtered out at room temperature to obtain a solution of the product, which was concentrated and acidified to obtain the product.
[0053] Liquid phase analysis showed that the product content was 98.2%, and the yield was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com