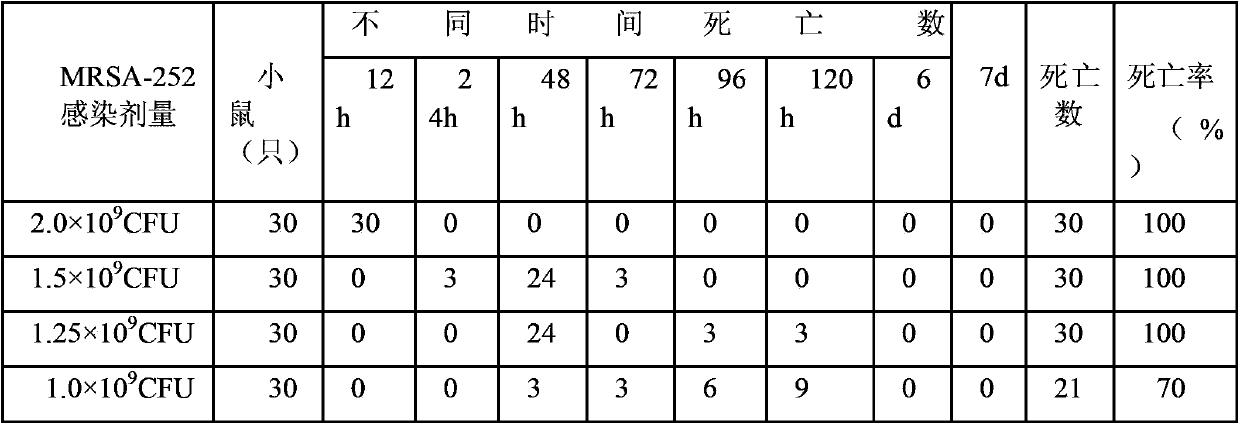
MRSA (Methicillin Resistant Staphylococcus Aureus) vaccine recombinant protein antigen I1C and preparation method and application thereof
A recombinant protein and protein technology, applied in biochemical equipment and methods, chemical instruments and methods, recombinant DNA technology, etc., can solve the problems of unfavorable vaccine industrialization preparation, cumbersome methods, and great difficulty, so as to maintain spatial conformation and immunity The effect of originality, simple preparation method and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
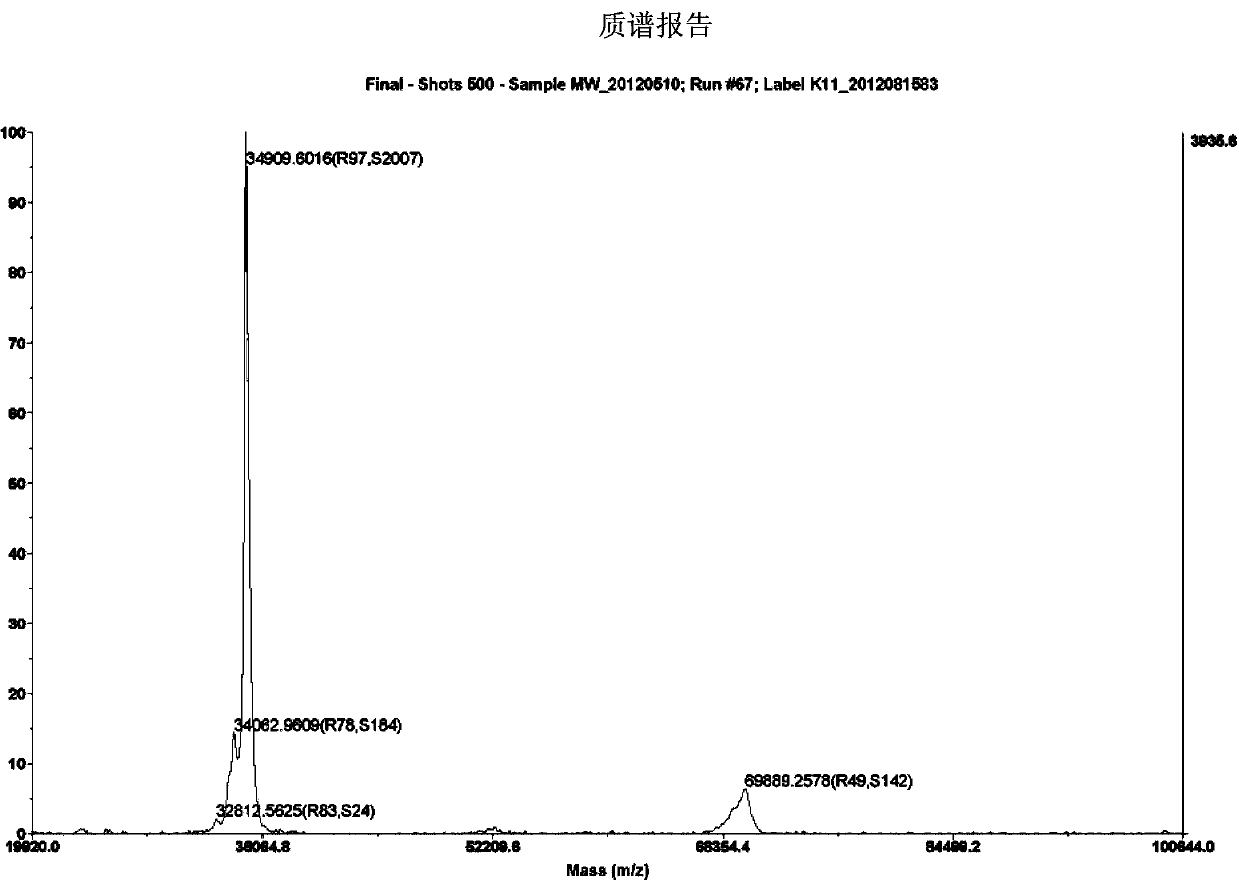
[0047] Example 1: The DNA sequence of I1C was synthesized by Shanghai Jierui Biotechnology Co., Ltd. and constructed on the vectors pGEX-6P-2 and pET22b, and the host bacteria XL-1blue, BL21(DE3), and HMS(174) were transformed respectively.
Embodiment 2
[0048] Example 2: Induced expression, purification and identification of expression forms of I1C multi-subunits in prokaryotic expression system-Escherichia coli
[0049] 1. Induced expression of target protein
[0050] 1) Take 100 μL of the pGEX-6P-2-I1C / XL-1blue bacterial solution that has been correctly identified by double enzyme digestion and add it to 10 mL of Amp-resistant TB medium, cultivate overnight at 80 rpm at 37°C, and take 400 μL of the overnight cultured bacterial solution and add 20 mL of Amp-resistant bacterial solution TB medium (the rest of the bacterial solution was stored in a refrigerator at 4°C for later use), cultured at 37°C for 2-3 hours at a rotation speed of 200 rpm, and after secondary activation until the OD600 was 0.6-0.8, 40 μL of IPTG was added to make the final concentration 200 μM. Then placed on a shaker to induce expression overnight at 16°C.
[0051] 2) Take out the bacterial liquid after induced expression, centrifuge at 6000rpm for 15m...
Embodiment 3
[0058] Embodiment 3: Preparation of I1C antigen
[0059] 1. Scale up culture to obtain protein
[0060] Take 400 μL of the spare pGEX-6P-2-I1C / XL-1blue bacterial solution stored in the refrigerator at 4°C and add it to 20mL TB medium containing Amp resistance for one activation. After culturing at 200rpm and 37°C for 5~6h, take 8mL once Add the activated bacterial solution to 400mL TB medium containing Amp resistance for secondary activation, culture at 37°C for 3~4h until the OD600 is 0.8, add 80μl IPTG (final concentration is 200uM) and place in a shaker at 16°C overnight After induction, centrifuge at 6000rpm for 15min to collect the bacteria, add 20mL of lysis buffer to resuspend the bacteria, then ultrasonically lyse the bacteria for 3min (200V), collect the supernatant and 800μL of Glutathione Sepharose4B gel beads for binding to the GST fusion protein ( beads) combined processing.
[0061] 2. Use the enzyme digestion method to separate the target protein from the GST...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com