A kind of preparation method and application of double Schiff base fluorescent polymer
A fluorescent polymer and bi-Schiff base technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effects of simple methods and equipment, low reaction temperature, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] Example 1: Synthesis of bi-Schiff base fluorescent polymer with fluorescent properties.
[0033] 1. After installing the reaction device, add 0.5366g of terephthalaldehyde, 30mL of methanol or DMF or DMSO to a three-necked flask or other reaction flask in sequence, then stir until the terephthalaldehyde is completely dissolved, then add 1.6mL of ammonia water to the bottle .
[0034] 2. Use a water bath to heat a three-neck flask or other reaction flask to 75°C, and then maintain a constant temperature under reflux for 12 hours.
[0035] 3. After the reaction is over, cool the three-neck flask or other reaction flasks to room temperature, filter the reaction mixture with suction, wash with methanol or ethanol several times, and dry it in a vacuum oven at 60°C. After 24 hours, the products P-meth, P -dmf or P-dmso.
example 2
[0036] Example 2: Test the ultraviolet absorption spectrum and fluorescence spectrum of the product obtained.
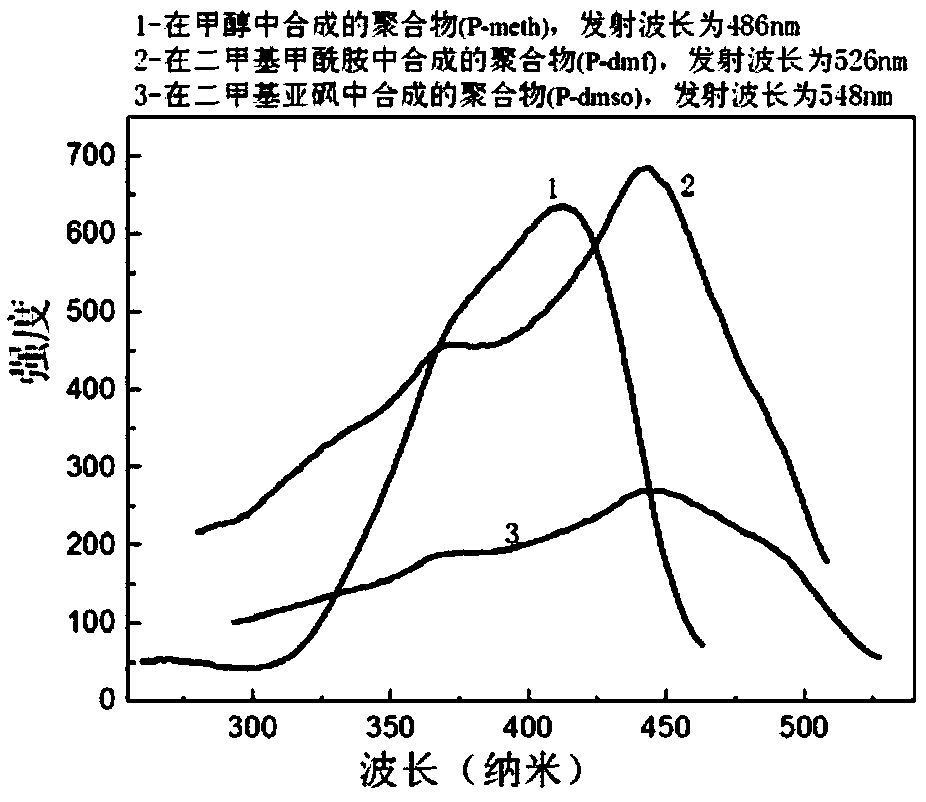
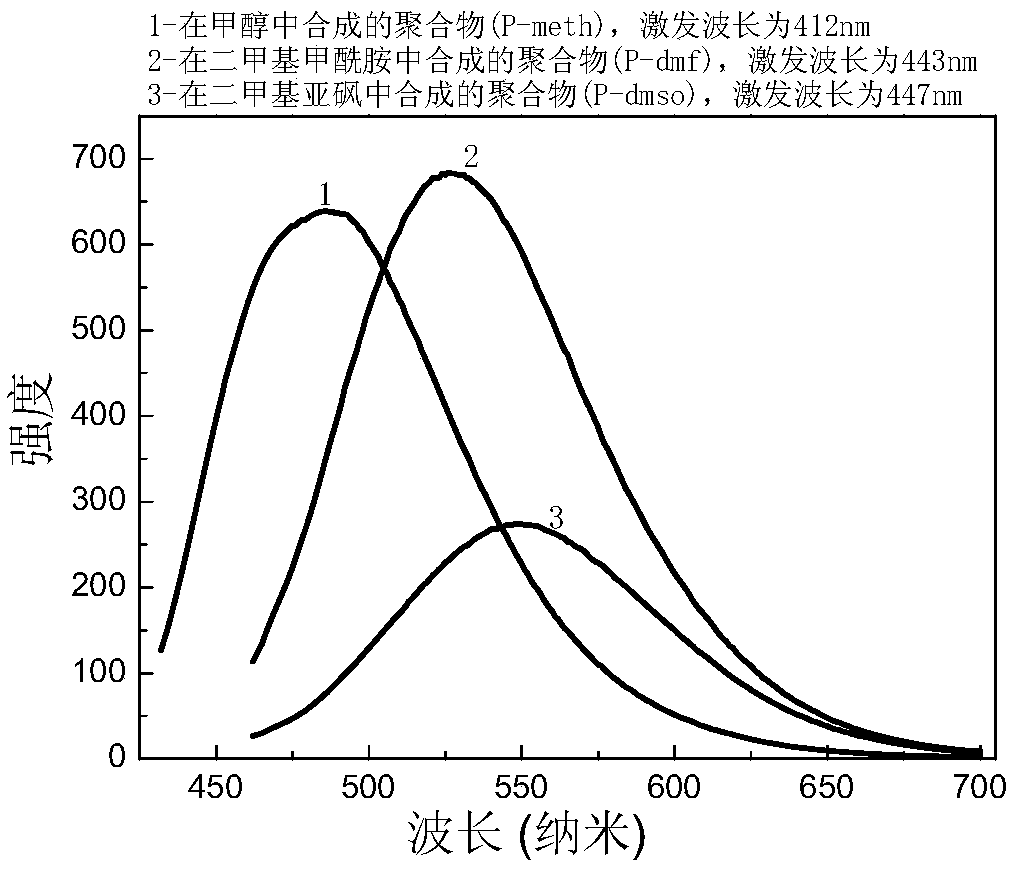
[0037] Depend on figure 1 The fluorescence excitation spectrum and figure 2 It can be known that the emission wavelengths of the samples are: P-meth 486nm (λex=412nm), P-dmf 526nm (λex=443nm), P-dmso 548nm (λex=447nm).
example 3
[0038]Example 3: Test the infrared spectrum and carbon nuclear magnetic resonance spectrum of the obtained sample.
[0039] The product is compared with terephthalaldehyde in infrared spectrum, and it is found that 1698cm -1 C=O stretching vibration absorption peak disappears, 1604cm -1 The appearance of C=N stretching vibration absorption peak indicates that the polycondensation reaction has occurred. From the carbon nuclear magnetic resonance spectrum, it can be seen that the peaks at 68 and 81ppm are the carbons of -CH-N, 131-150ppm are the carbons on the benzene ring, 166 and 181ppm are the carbons of -C=N, and 195ppm are the carbons on the terminal aldehyde group carbon.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com