Orally disintegrating tablet containing sertraline hydrochloride and preparation method thereof
A technology of sertraline hydrochloride and orally disintegrating tablets, which is applied to medical preparations containing active ingredients, medical preparations without active ingredients, and pharmaceutical formulas, which can solve the problem of difficulty in achieving taste-masking effects, high cost, and unfavorable industrial production And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
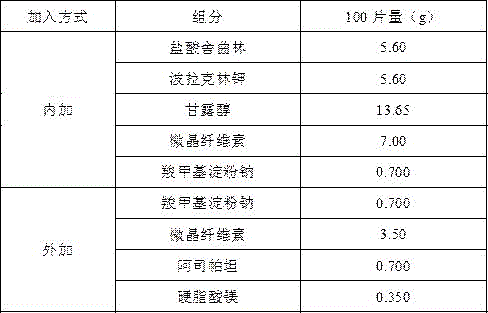
Embodiment 1
[0012]
[0013] Preparation Process
[0014] Magnetically stir sertraline hydrochloride and polacrilin potassium in 40 times the weight of purified water for 3 hours, centrifuge at 4000rpm for 10 minutes, collect the precipitate and dry it at 50°C, grind it, add microcrystalline cellulose, mannitol and sodium carboxymethyl starch to pass through 80 mesh Sieve 3 times and mix well, use water as binder, granulate with 24-mesh sieve, blast dry at 50°C, dry to 1-3% moisture, pass the dried granules through 24-mesh sieve for granulation, weigh, and convert yield, Add sodium carboxymethyl starch, microcrystalline cellulose, aspartame and magnesium stearate, Φ10 flat punched tablets, tablet weight 350mg, hardness 20-60N. The preparation prepared by the prescription is sweet in the mouth, bitter, numb and spicy in aftertaste, and the oral disintegration time is greater than 60 s.
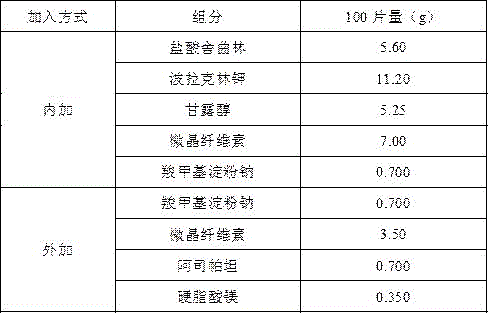
Embodiment 2
[0016]
[0017] Preparation Process
[0018] Magnetically stir sertraline hydrochloride and polacrilin potassium in 40 times the weight of purified water for 4 hours, centrifuge at 4000rpm for 10 minutes, collect the precipitate and dry it at 50°C, grind it, add microcrystalline cellulose, mannitol and sodium carboxymethyl starch to pass through 80 mesh Sieve 3 times and mix well, use water as binder, granulate with 24-mesh sieve, blast dry at 50°C, dry to 1-3% moisture, pass the dried granules through 24-mesh sieve for granulation, weigh, and convert yield, Add sodium carboxymethyl starch, microcrystalline cellulose, aspartame and magnesium stearate, Φ10 flat punched tablets, tablet weight 350mg, hardness 20-60N. The preparation prepared by the prescription is sweet in the mouth, without bitterness, numbness and spicy feeling, and the oral disintegration time is less than 60s.
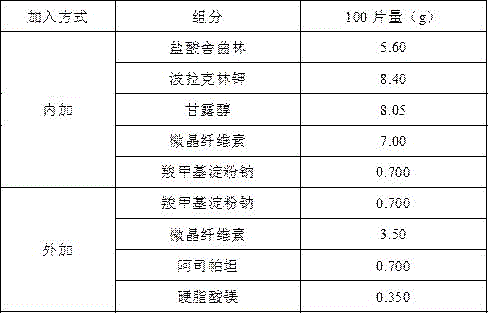
Embodiment 3
[0020]
[0021] Preparation Process
[0022] Magnetically stir sertraline hydrochloride and polacrilin potassium in 20 times the weight of purified water for 4 hours, centrifuge at 4000rpm for 10 minutes, collect the precipitate and dry it at 50°C, grind it, add microcrystalline cellulose, mannitol and sodium carboxymethyl starch to pass through 80 mesh Sieve 3 times and mix well, use water as binder, granulate with 24-mesh sieve, blast dry at 50°C, dry to 1-3% moisture, pass the dried granules through 24-mesh sieve for granulation, weigh, and convert yield, Add sodium carboxymethyl starch, microcrystalline cellulose, aspartame and magnesium stearate, Φ10 flat punched tablets, tablet weight 350mg, hardness 20-60N. The preparation prepared by this prescription is sweet in the mouth, slightly bitter, numb and spicy in aftertaste, and the oral disintegration time is greater than 60s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com