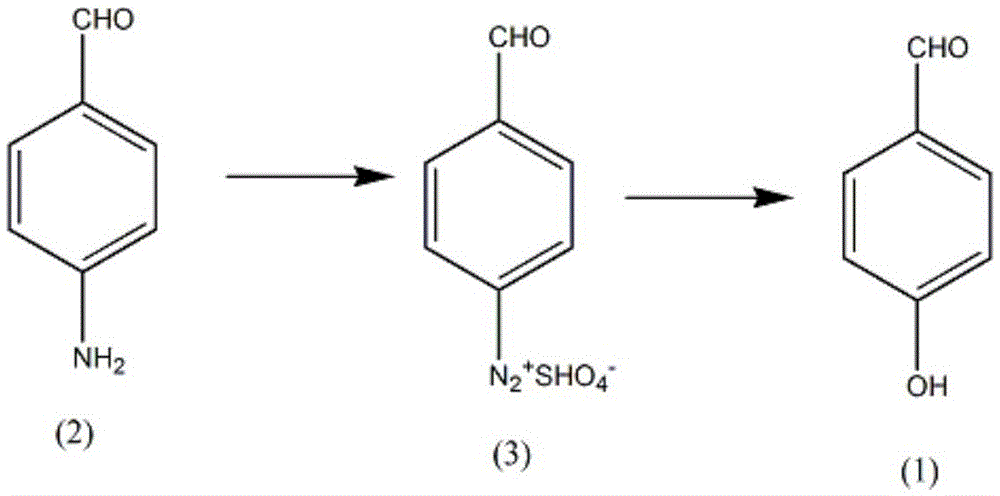
Synthetic method for trimethoprim drug intermediate-p-hydroxy benzaldehyde
A technology of p-hydroxybenzaldehyde and trimethoprim, which is applied in the field of synthesis of trimethoprim drug intermediate p-hydroxybenzaldehyde, can solve the problems of rarely used alone, and bacteria are prone to drug resistance, so as to reduce intermediate link, reduce the reaction temperature and reaction time, and increase the effect of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0014] (i) Add 0.17mol of p-aminobenzaldehyde (2) and 150ml of water into a reaction vessel with a volume of 800mL, slowly add 50mL of concentrated phosphoric acid with a mass fraction of 50%, control the temperature of the solution to rise to 80°C, and generate phosphate to obtain Suspension, cool the solution to 15°C, slowly add 0.25mol of sodium bisulfite dissolved in 100ml of aqueous solution dropwise, control the stirring speed at 100rpm during the dropwise addition, and test the end point with potassium iodide test paper;
[0015] (ii) Control the temperature of the solution at 30°C, let it stand for 20min, add 5g of urea, control the temperature of the solution to rise gradually, after a large amount of gas escapes, heat the solution to 90°C, keep it for 10min, decolorize with molecular sieve, filter while hot, filter The cake was washed with ether solvent, the filtrate and the washing liquid were combined, and the solid was precipitated after the temperature was lowered...
example 2
[0017] (i) Add 0.17mol of p-aminobenzaldehyde (2) and 180ml of water into a reaction vessel with a volume of 900mL, slowly add 50mL of concentrated phosphoric acid with a mass fraction of 60%, control the temperature of the solution to rise to 90°C, and generate phosphate, and obtain Suspension, cool the solution to 17°C, slowly add 0.25mol of sodium bisulfite dissolved in 100ml of aqueous solution dropwise, control the stirring speed at 200rpm during the dropwise addition, and test the end point with potassium iodide test paper;
[0018] (ii) Control the temperature of the solution at 30°C, let it stand for 20min, add 5g of urea, control the temperature of the solution to rise gradually, after a large amount of gas escapes, heat the solution to 92°C, keep it for 10min, decolorize with molecular sieve, filter while hot, filter The cake was washed with acetone solvent, the filtrate and the washing liquid were combined, and the solid was precipitated after the temperature was low...
example 3
[0020] (i) Add 0.17mol of p-aminobenzaldehyde (2) and 200ml of water into a reaction vessel with a volume of 1000mL, slowly add 50mL of concentrated phosphoric acid, control the temperature of the solution to rise to 95°C, and generate phosphate to obtain a suspension, and cool the solution To 20°C, slowly add 0.25mol of sodium bisulfite dissolved in 100ml of aqueous solution dropwise, control the stirring speed at 300rpm during the dropwise addition, and test the end point with potassium iodide test paper;
[0021] (ii) Control the temperature of the solution at 30°C, let it stand for 20min, add 5g of urea, control the temperature of the solution to rise gradually, after a large amount of gas escapes, heat the solution to 95°C, keep it for 10min, decolorize with molecular sieve, filter while hot, filter The cake was washed with ethyl acetate solvent, the filtrate and the washing liquid were combined, and a solid was precipitated after the temperature was lowered, and the solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com