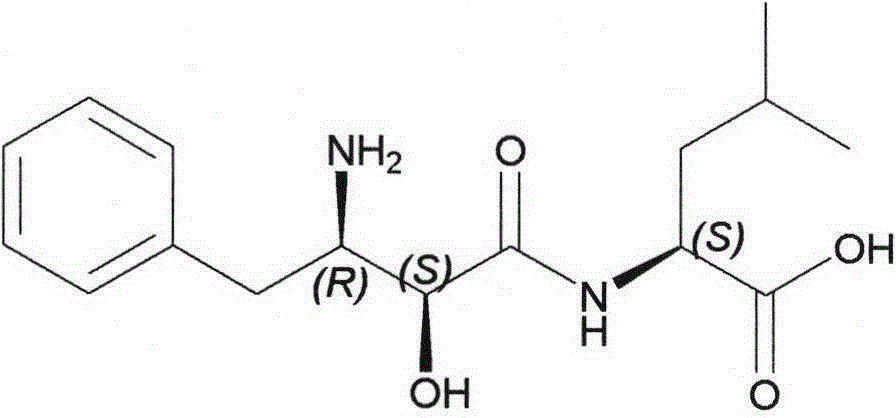
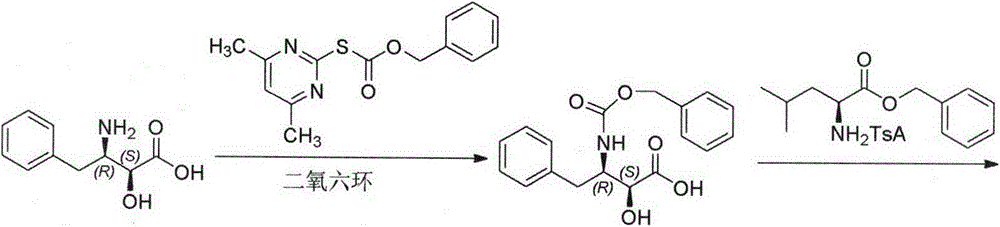
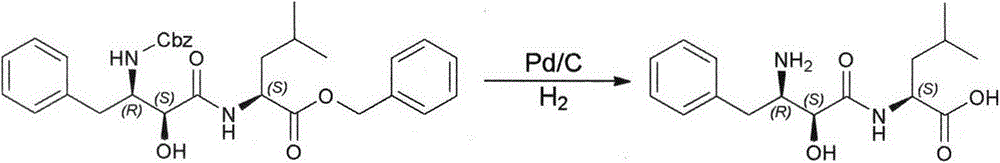
Method for synthesizing Ubenimex
A synthesis method, the technology of Ubenex, is applied in chemical instruments and methods, preparation of organic compounds, production of bulk chemicals, etc. Mass production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of (2S, 3R)-N-Boc-3-amino-2-hydroxyl-4-phenylbutyric acid (3)
[0023]
[0024] Add 39.0g (0.2mol) of 3-amino-2-hydroxy-4-phenylbutyric acid, 200mL of water, 400mL of acetone to the reaction flask, add 42mL of triethylamine (0.3mol), stir to completely dissolve, add dicarbonic acid 48.0 g (0.22 mol) of di-tert-butyl ester was stirred at room temperature for reaction. The reaction solution was concentrated under reduced pressure to remove most of the solvent, and after cooling, it was adjusted to acidity with hydrochloric acid, extracted with ethyl acetate, and dried over anhydrous sodium sulfate.
[0025] The desiccant was filtered off, spin-dried under reduced pressure, and viscous matter remained. Add 60 mL of ethyl acetate, heat to dissolve, then add 360 mL of preheated n-hexane, and cool to crystallize. Filter, wash with ethyl acetate / n-hexane, and dry to obtain (3) 38g, mp: 128-130°C, yield 64%, content 98.7% (HPLC normalization method). ...
Embodiment 2
[0026] Example 2: Preparation of N-[(2S, 3R)-N-Boc-3-amino-2-hydroxyl-4-phenylbutyryl]-L-leucine tert-butyl ester
[0027]
[0028] Add 35.4 g (0.12 mol) of Boc protected compound (3) and 400 mL of dichloromethane into the reaction flask, then add 32.4 g (0.144 mol) of L-leucine tert-butyl ester hydrochloride, 1-hydroxybenzotriazepam Dissolve azole 19.5g (0.144mol) and triethylamine 20.2mL (0.144mol). After cooling to 0°C, 32.1 g (0.156 mol) of DCC solution was added dropwise, and a white solid appeared shortly after the addition, and stirred at room temperature for 4 hours after the addition. Add 200 mL of dilute hydrochloric acid and stir for 20 minutes, filter, wash the filtrate with dilute sodium hydroxide solution and water, and dry over anhydrous sodium sulfate.
[0029] The solid was filtered off, the filtrate was spin-dried under reduced pressure, the residue was heated and dissolved with 60 mL of ethyl acetate, 300 mL of preheated n-hexane was added, cooled and cr...
Embodiment 3
[0030] Embodiment 3: the preparation of Ubenimex
[0031]
[0032] Add 50 g (0.108 mol) of the condensate (4) to the reaction flask, add 250 mL of 4 M hydrogen chloride / acetone solution under stirring, dissolve immediately, and then precipitate a solid, and continue the reaction overnight.
[0033] Filter and fully soak with acetone to obtain a white powder, which is dried to obtain 31g. Dissolve in 620 mL of water, add an appropriate amount of activated carbon for decolorization, filter, adjust the pH of the filtrate to 5-6 with concentrated ammonia water, and precipitate a white solid, filter, wash with ice water, and dry to obtain 25.2 g of (1), with a yield of 76%. Detected by HPLC, the content is 99.68% (normalized method), and the maximum single impurity is 0.05%. ( (c=1.0in0.1NHCl); Elemental analysis: C%=62.37, N%=9.18, H%=7.86, theoretical value: C%=62.32, N%=9.08, H%=7.84; IR (KBr): 2956, 1694, 1641, 1541cm -1 ; MS-ESI: (M+Na) + = 330.2; 1 HNMR (500MHz, DMS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com