Synthesis method for marine natural product Puupehenone
A natural product and synthetic method technology, applied in organic chemistry and other fields, can solve the problems of long synthetic route, low yield, poor stereoselectivity, etc., and achieve the effect of less reaction steps, high total yield and good product selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
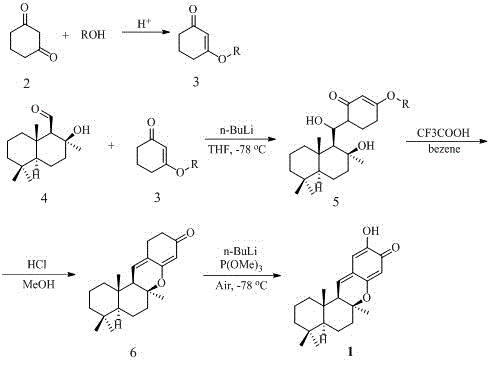
[0019] Embodiment 1: the synthesis of ethoxycyclohexanedione (3, see accompanying drawing)
[0020] Take 1.12 g of cyclohexanedione (2, 10.0 mmol) and dissolve it in 25 ml of absolute ethanol, add p-toluenesulfonic acid (1.0 mmol), stir and react at room temperature for 3 hours, and TLC detects that the reaction is complete. Add 30 ml of water, extract with dichloromethane (30mLx3), combine the organic phases, wash with saturated sodium bicarbonate solution, dry over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to obtain a light yellow oily substance ethoxy-protected ring 1.23 grams of hexanedione, the yield is 88%.
Embodiment 2
[0021] Example 2: Synthesis of beta-hydroxycyclohexanone (5, see accompanying drawing) of bicyclic diterpene
[0022] Dissolve 140 mg (3, 1.0 mmol) of ethoxy-protected cyclohexanedione in 2 ml of anhydrous tetrahydrofuran, repeatedly fill and exhaust argon three times, exhaust the air, and cool down to -78 o c. LDA (3.0 mmol) was added and the reaction was kept stirring at this temperature for 30 minutes. Another 238 mg of (-) sclarealdehyde (4, see attached figure) was dissolved in 1 ml of anhydrous THF, slowly added dropwise to the above reaction system, and the stirring reaction was continued for 12 hours, and the reaction was detected by TLC. 5 ml of saturated ammonium chloride solution was added dropwise to the reaction system to quench the reaction, and stirring was continued at room temperature for 30 minutes. Extracted with ethyl acetate (10mLx3), combined organic phases, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and pu...
Embodiment 3
[0023] Embodiment 3: the synthesis of the basic skeleton (6, see accompanying drawing) of Puupehenone
[0024] Dissolve 189 mg (5,0.5 mmol) of beta-hydroxycyclohexanone of bicyclic diterpene in 4 ml of benzene, add trifluoroacetic acid (0.5 mmol) and stir for reaction at room temperature for 1 hour, and TLC detects that the reaction is complete. Add 10 ml of ethyl acetate, wash with saturated brine (10 mL x 3), dry the organic phase over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to obtain 129 mg of white solid with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com