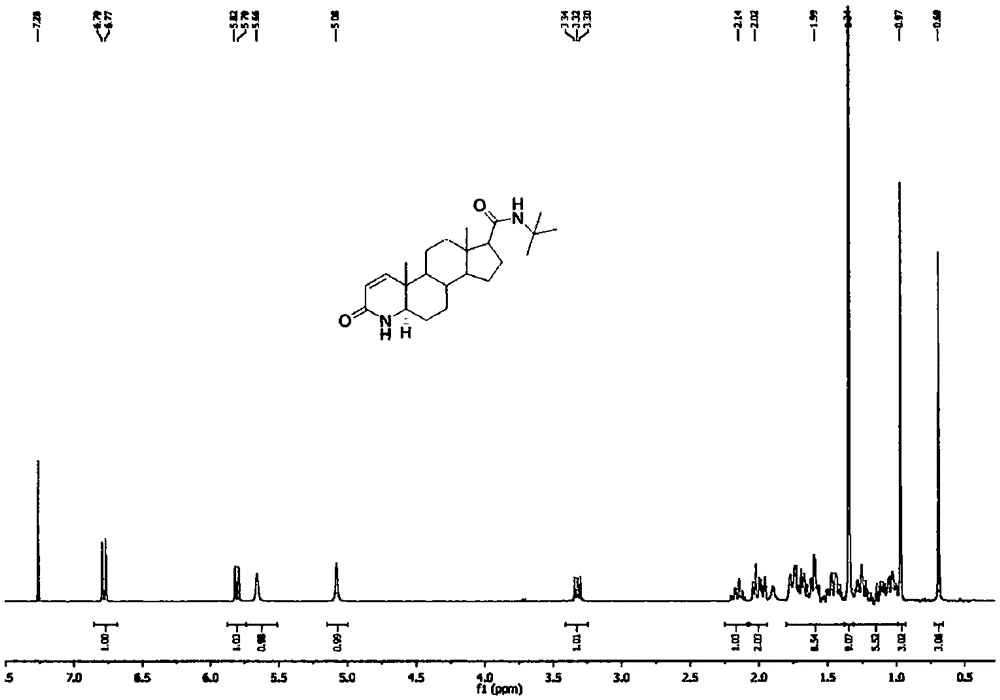
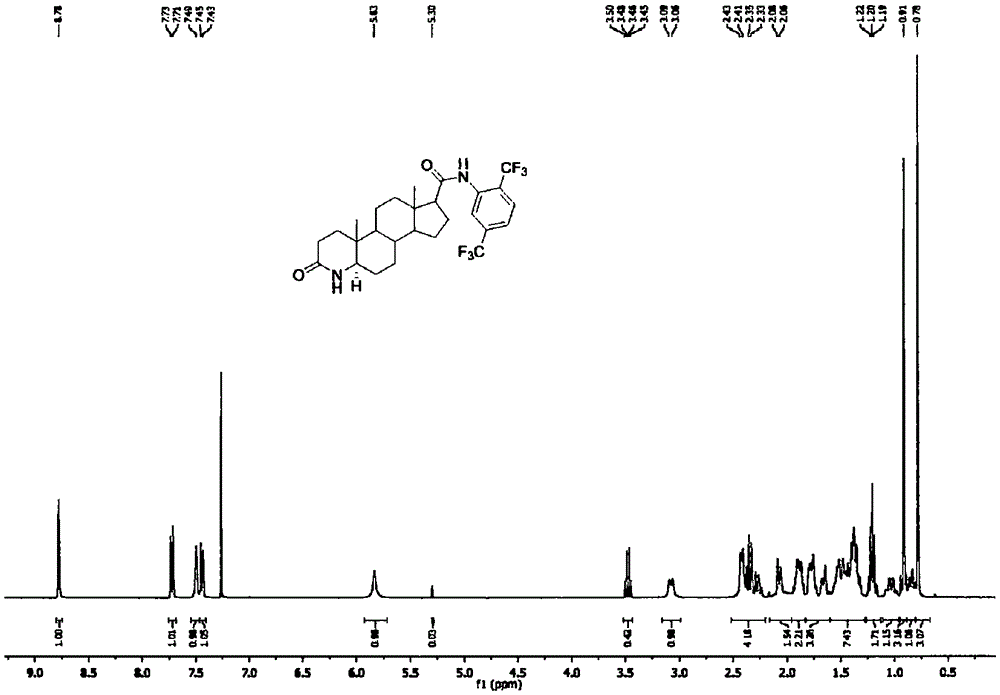
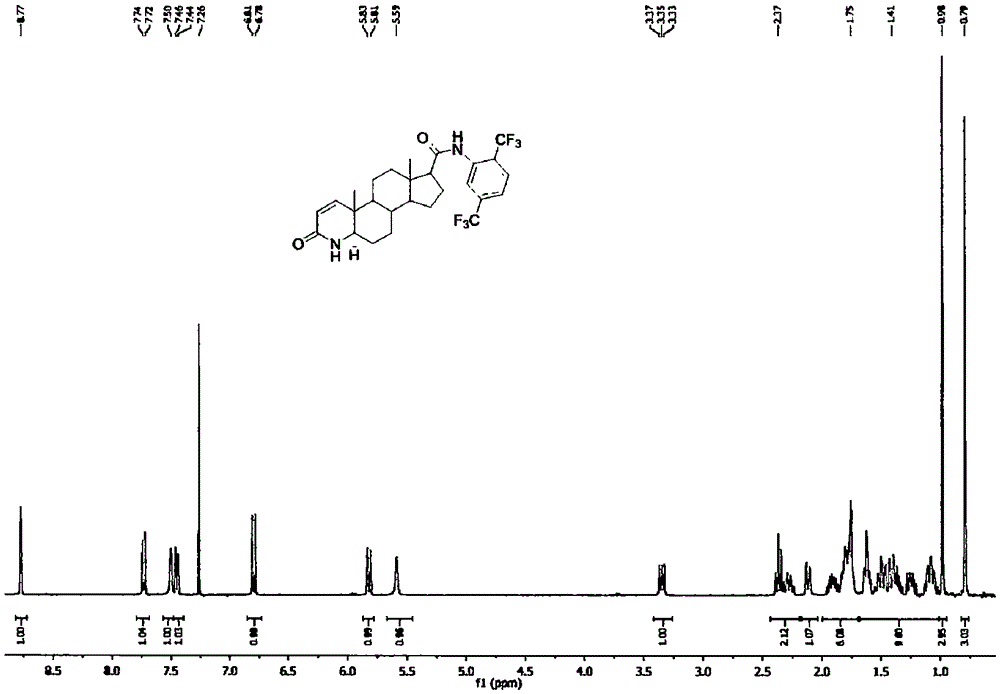
Method for forming double bonds between 1-position and 2-position during synthesis of finasteride and dutasteride
A technology of dutasteride and compound salt, which is applied in the field of drug synthesis, can solve the problems of difficult refining, product quality decline, long reaction time, etc., and achieve the effect of good product color, mild reaction conditions, economical and environmentally friendly process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of embodiment 1 dihydrofinasteride iodide
[0035] Under the protection of nitrogen atmosphere, 30 g of dihydrofinasteride was dissolved in 300 ml of dry dichloromethane, and then 36 ml of dry N,N,N,N-tetramethylethylenediamine was added. The reaction mixture was cooled to 0°C, and 20.3 ml of trimethylchlorosilane was added. A white ammonium salt precipitate then precipitated out and the reaction mixture was stirred for an additional 20 minutes. The 24.4 g of iodine was added in three additions at 10-minute intervals. Stirring was continued for 1 hour at 0 to 5°C until the reaction of dihydrofinasteride was complete. The reaction was followed by 50 ml of 10% Na 2 SO 3The solution was quenched with 50 ml of saturated sodium chloride solution. After adding 50 ml of toluene to the separated organic phase, it was concentrated to 60 ml under reduced pressure at less than 40°C. 150 ml of petroleum ether was slowly added dropwise to the above stirred concen...
Embodiment 2
[0037] The synthesis of embodiment 2 dihydrofinasteride iodide
[0038] Under the protection of nitrogen atmosphere, 10.00 g of dihydrofinasteride was dispersed in 100 ml of dry toluene, and then 12.0 ml of dry N,N,N,N-tetramethylethylenediamine was added. The reaction mixture was cooled to 0°C, and 6.76 ml of trimethylchlorosilane was added. Dihydrofinasteride was gradually dissolved, and a white ammonium salt precipitate was precipitated out, and the reaction mixture was stirred for 30 minutes. 8.14 grams of iodine was added three times every 20 minutes. Stirring was continued at 0 to 5°C for 2 hours until the reaction of dihydrofinasteride was complete. The reaction was then quenched with 50 mL of 10% Na2SO3 solution. Stirring was continued for about 1 hour, and the product slowly precipitated out from the reaction mixture. Subsequently, 50 milliliters of petroleum ether was added dropwise to the reaction mixture to fully precipitate the product. Stirring was continued...
Embodiment 3
[0040] The synthesis of embodiment 3 dihydrodutasteride
[0041] Dissolve 16.65 g of 3-keto-4-aza-5α-androst-17β-carboxylate methyl ester in 250 ml of dichloromethane, and add 18.95 ml of boron tribromide dropwise under cooling with room temperature water (25°C) . Stirring was continued at room temperature for 20 minutes to obtain a clear brown solution. 19.5 ml of 2,5-bis(trifluoromethyl)aniline was added to the above solution under cooling with water at room temperature (25°C). The reaction was heated to 50 °C and stirred overnight. After the reaction solution was cooled to room temperature, 50 milliliters of water was slowly added, and the hydrogen bromide gas generated was introduced into Na 2 CO 3 in aqueous solution. Continue to add 200 ml of water until all solids are dissolved and two clear phases are obtained. After the dichloromethane organic phase of the lower layer was separated and washed with 200 ml of water and 200 ml of saturated brine, anhydrous Na 2 SO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com