Composition of human adipose derived mesenchymal progenitor cells and adipose derived stromal vascular fraction for treating hepatitis B
A technology of stromal vascular components and stromal vascular components, applied in peptide/protein components, drug combinations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
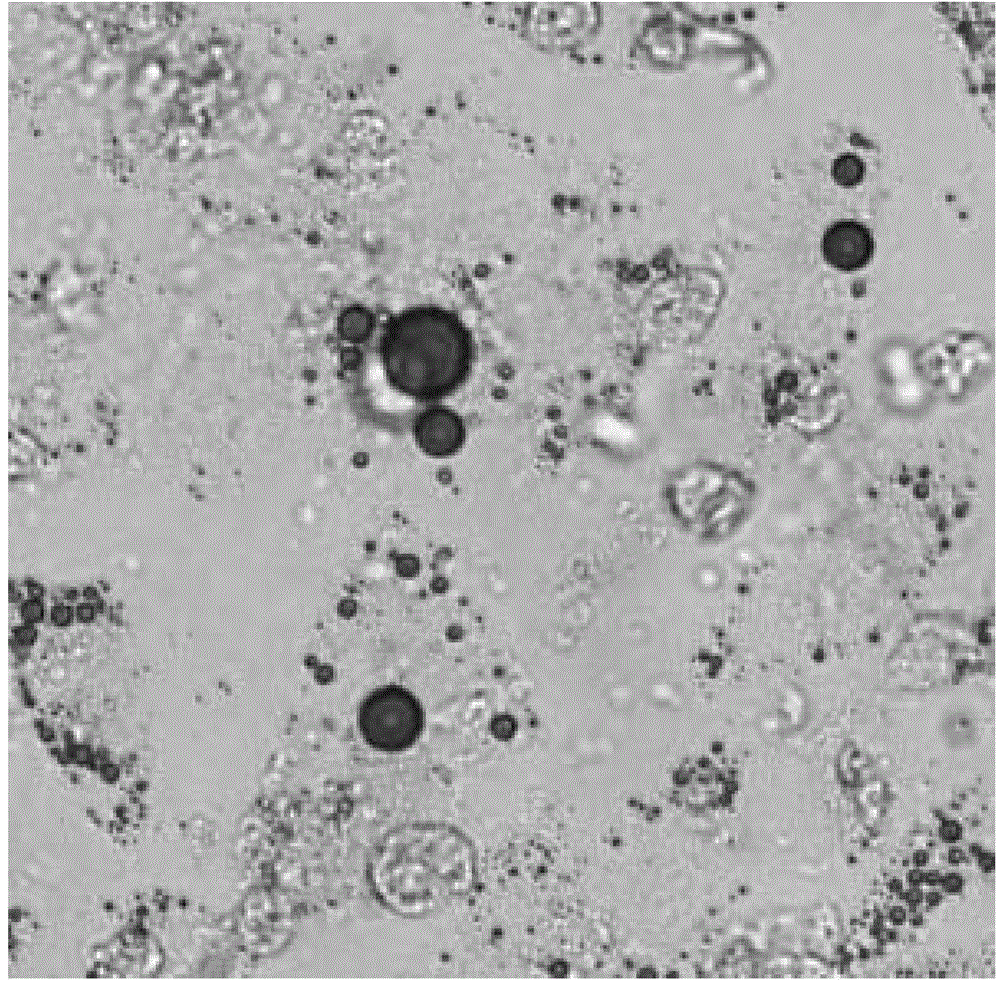
[0098] In the present invention, the preparation method of adipose mesenchymal progenitor cells may include the steps of: washing adipose tissue, then digesting it with collagenase, centrifuging to separate stromal and vascular components, removing oil and collagenase, culturing primary cells, and obtaining passaged adipose tissue mesenchymal progenitor cells.
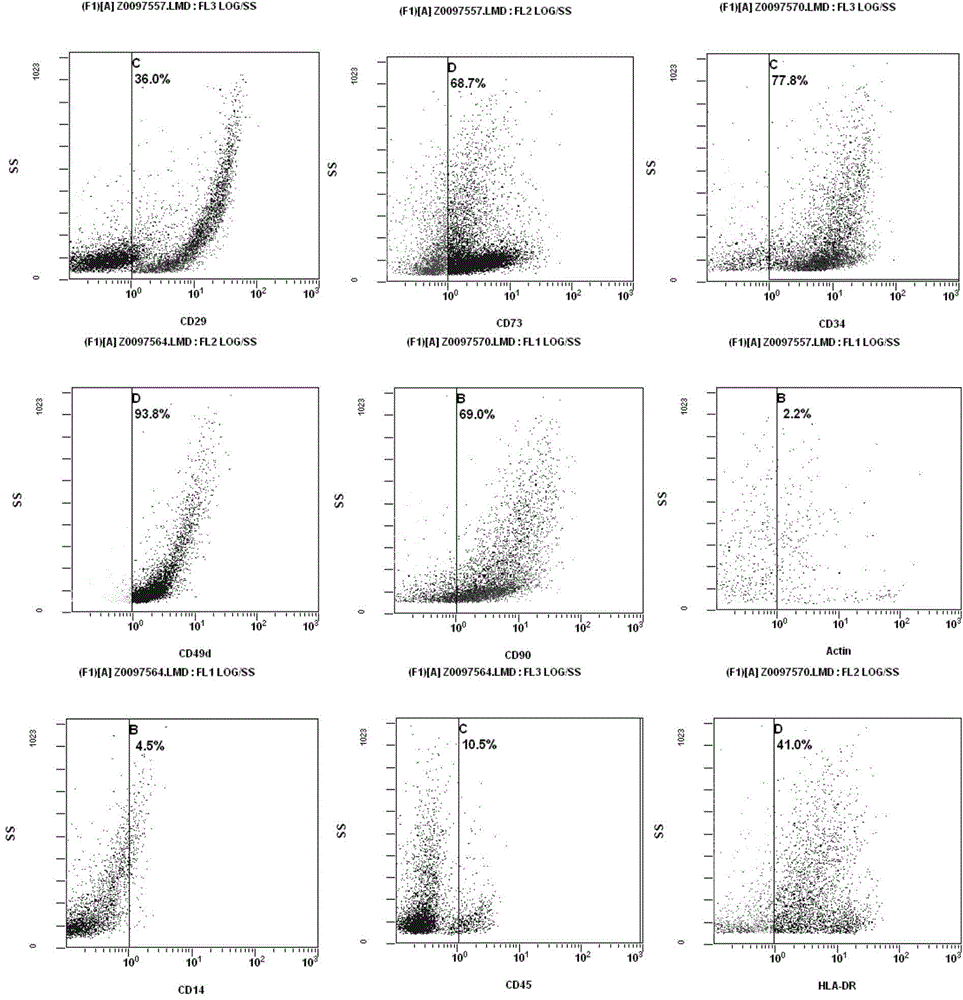
[0099] Antigen detection of adipose-derived mesenchymal progenitor cells
[0100] The adipose-derived mesenchymal progenitor cells used in the present invention have high purity and basically do not contain other types of cells or stem cells. This can be verified by detection of cell surface antigens.
[0101] Adipose mesenchymal progenitor cells have a variety of specific antigens and receptors, mainly including CD3, CD13, D29, CD34, CD45, CD49e, CD59, CD73, CD90, CD105, HLA-ABC, etc.
[0102] CD34 antigen is a highly glycosylated type I transmembrane protein, which is selectively expressed on the surface of human h...
Embodiment 1
[0123] The preparation of embodiment 1haMPC
[0124] 1. Reagents and consumables
[0125] 1. Sterile surgical instruments and consumables
[0126] (1) 5 sterile long-handled surgical forceps
[0127] (2) Sterile 100 mesh filter
[0128] (3) Sterilized 40-mesh filter
[0129] (4) 50ml centrifuge tube
[0130] (5) T175, T75 culture flask
[0131] (6) T10ml, T25ml pipettes
[0132] (7) Wide mouth tip pipette
[0133] 2. Sterile reagents:
[0134] (1) DMEM (serum-free medium), MSCSFM medium (life);
[0135] (2) Collagenase type I (ready to use): 0.1% collagenase I Preparation method: Weigh 0.1g collagenase I powder and dissolve it in 100ml medium without adding any factors, and preheat at 37°C before use;
[0136] (3) Sodium chloride injection;
[0137] (4) 0.125% Trypsin-0.01% EDTA solution.
Embodiment approach
[0139] 1. To receive adipose tissue, wipe the outer wall of the container containing adipose tissue with 75% alcohol;
[0140] 2. Dispense adipose tissue, each T175 culture bottle is divided into 50ml adipose tissue. With a 10ml pipette, remove the tip, first absorb the lower layer of red liquid into the fat collection bottle and discard it, and mix the remaining upper layer of fat before subpackaging.
[0141] 3. Wash the adipose tissue to remove blood cells. Add 100ml sodium chloride injection to the T175 culture bottle, tighten the cap, shake vigorously for 3 minutes to fully wash the adipose tissue, then stand still for 3-5 minutes to separate the different phases, and suck off the lower aqueous phase; repeat the above operation three times until The lower layer became clear.
[0142] 4. Collagenase I digestion: Add an equal amount of freshly prepared collagenase I solution (preheated on an air bath shaker at 37°C half an hour in advance), seal with a parafilm, shake the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com