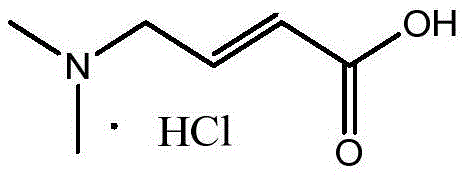
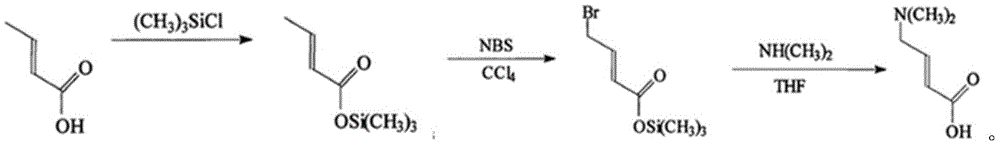
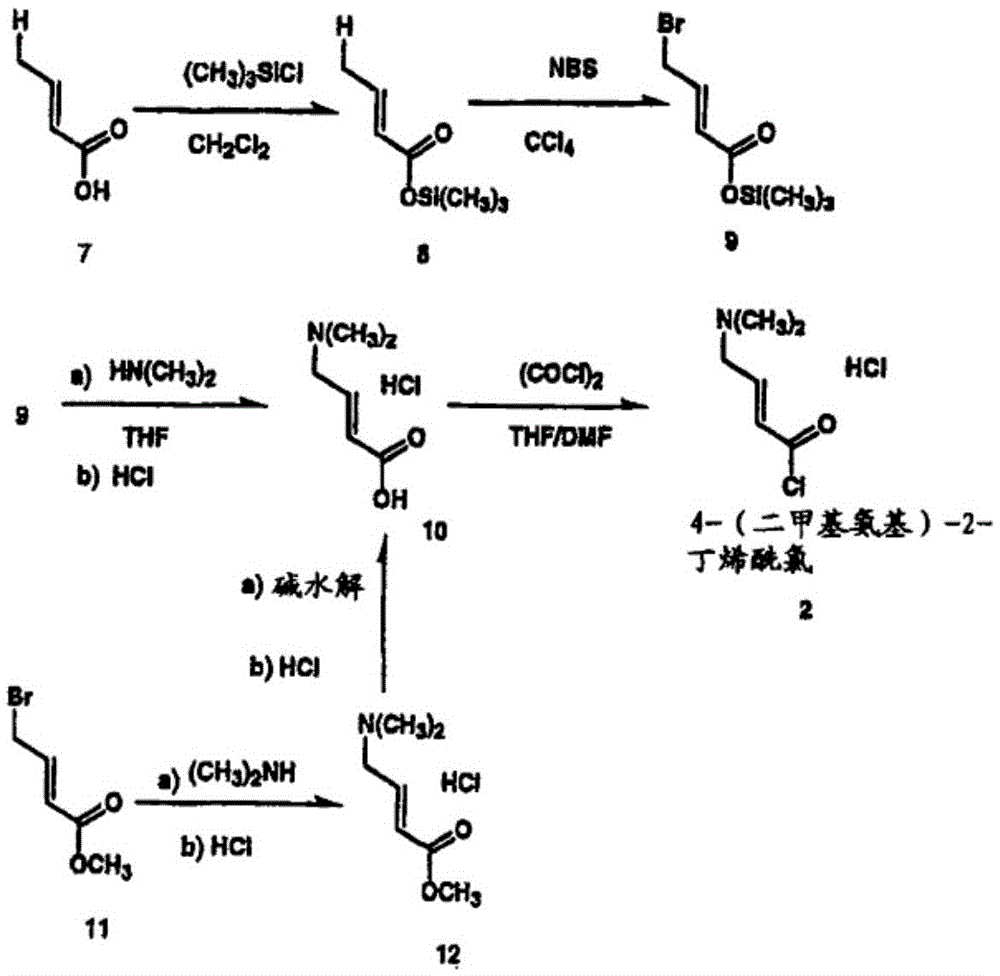
Method for preparing highly pure trans-4-dimethylaminocrotonic acid hydrochloride through one-pot technology
A technology of dimethylamino crotonate hydrochloride and dimethylamino crotonate is applied in the field of preparing high-purity trans-4-dimethylamino crotonate hydrochloride by one-pot method, which can Solve the problems of complex process and low product purity, and achieve the effect of simple operation steps, good product purity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] To prepare trans-dimethylaminocroton hydrochloride, follow the steps below:
[0030] 1. Preparation of trans-4-bromocrotonate
[0031] At room temperature, 50.0g (0.4995mol) trans-methyl crotonate, 89.3g (0.50mol) bromosuccinimide, 3.5g (0.01mol) benzoyl peroxide were successively put into a 1000ml four-necked reaction flask and 250.0ml chloroform. After feeding, start stirring until reflux for 10.0 hr, then cool down to room temperature to obtain trans-4-bromocrotonate methyl chloroform solution.
[0032] 2. Preparation of trans-4-dimethylaminocrotonic acid
[0033] At room temperature, slowly add 100.0 g of 33% dimethylamine aqueous solution (0.7320 mol) dropwise into the four-necked reaction flask in step 1, the dropping temperature is -10-25°C, the dropping time is 0.5 h, after the dropping is completed, stir and react for 0.5 hr, to obtain trans-4-dimethylamino crotonate methyl chloroform solution.
[0034] 3. Preparation of trans-4-dimethylaminocroton hydrochl...
Embodiment 2
[0037] To prepare trans-dimethylaminocroton hydrochloride, follow the steps below:
[0038] 1. Preparation of trans-4-bromocrotonate
[0039] At room temperature, 11.4g (0.1mol) trans-ethyl crotonate, 26.7g (0.15mol) bromosuccinimide, 0.93g (0.004mol) benzoyl peroxide were successively put into a 500ml four-necked reaction flask and 114.0 ml ethyl acetate. After feeding, start stirring until reflux for 15.0 hr, then cool down to room temperature to obtain trans-4-bromocrotonate ethyl chloroform solution.
[0040] 2. Preparation of trans-4-dimethylaminocrotonic acid
[0041] At room temperature, slowly add 40.8g of 33% dimethylamine aqueous solution (0.30mol) dropwise to the four-necked reaction flask in step 1, after the drop is completed, stir and react for 1.0hr to obtain trans-4-dimethylaminocrotonate ethyl ester Chloroform solution.
[0042] 3. Preparation of trans-4-dimethylaminocroton hydrochloride
[0043] At room temperature, slowly add 200ml of 1mol / L potassium h...
Embodiment 3
[0045] To prepare trans-dimethylaminocroton hydrochloride, follow the steps below:
[0046] 1. Preparation of trans-4-bromocrotonate
[0047] At room temperature, 20.0g (0.1560mol) trans isopropyl crotonate, 40.0g (0.2248mol) bromosuccinimide, 1.4g (0.0058mol) benzyl peroxide were successively put into a 1000ml four-necked reaction flask Acyl and 100.0ml carbon tetrachloride. After feeding, start stirring until reflux for 12.0 hr, then cool down to room temperature to obtain trans-4-bromocrotonate methyl chloroform solution.
[0048] 2. Preparation of trans-4-dimethylaminocrotonic acid
[0049] At room temperature, slowly drop 40.0 g of 33% dimethylamine aqueous solution (0.2928 mol) into the four-necked reaction flask in step 1, after the dropping, stir and react for 1.5 hr to obtain trans-4-dimethylaminocrotonate methyl ester Chloroform solution.
[0050] 3. Preparation of trans-4-dimethylaminocroton hydrochloride
[0051]At room temperature, slowly add 160ml of 1mol / L ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com