Glycine-L-cysteine-L-phenylalanine tripeptide and application thereof to preparation of medicines for treating stroke
A technology of cysteine and phenylalanine, applied in the field of medicine, can solve the problems of central nervous system adverse reactions, toxicity, side effects, and poor effect, and achieve the effect of improving cerebral infarction volume and treating stroke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
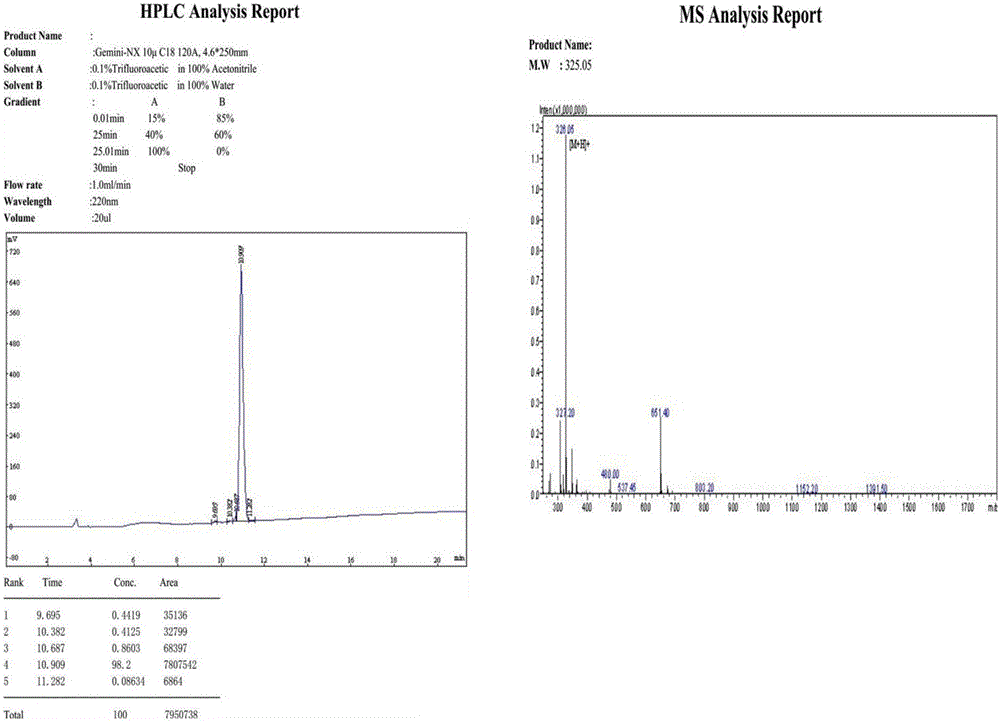
[0019] [Example 1] The preparation process of glycine-L-cysteine-L-phenylalanine tripeptide (Gly-Cys-Phe) is as follows:
[0020] 1. Synthesis of complex IFmoc-L-Phe-resin:
[0021] The tripeptide of this patent uses the method of solid-phase synthesis, weighs 10.7 g of 2-chlorotrityl chloride resin (2-ChlorotritylChloride Resin) with a degree of substitution of 0.4 mmol / g, puts the resin into a polypeptide reaction tube, and adds dichlorotrityl chloride Methane (DCM) 160.5ml, shaken for 30 minutes; filter the DCM solvent through the sand core, add 12mmol of Fmoc-L-Phe-OH amino acid, and then add 40mmol of N,N-diisopropylethylamine (DIEA), Finally, a small amount of dimethylformamide (DMF) was added to dissolve, and shaken for 1 h. Alternately wash 6 times with DMF and DCM. Add 160.5ml of 20% piperidine (piperidine is dissolved in DMF solution), take out the piperidine after 5 minutes, add 160.5ml of 20% piperidine again, wait for 15 minutes, take out the piperidine solution...
Embodiment 2
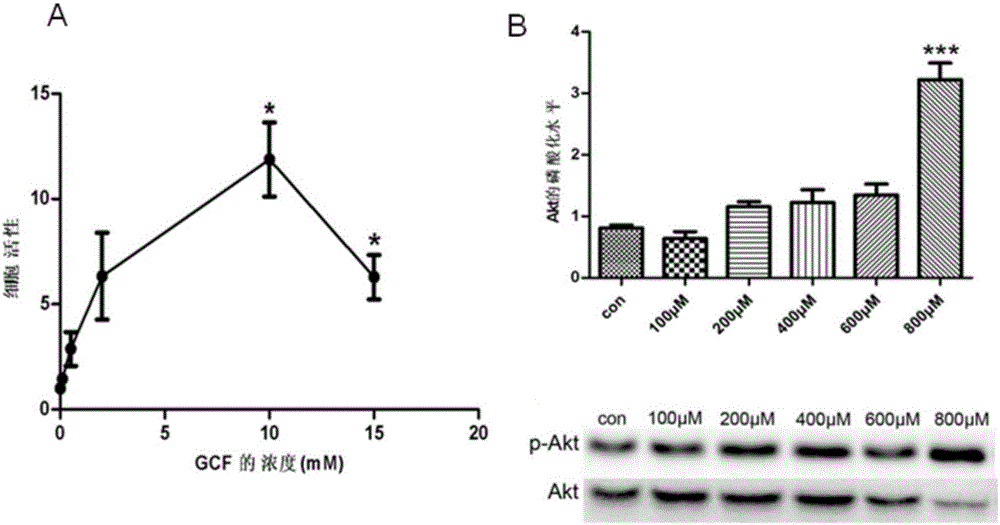
[0036] [Example 2] Cytotoxicity test of GCF on primary cortical neurons
[0037] In this example, the primary cortical neurons cultured for 10 days were used as the experimental object. After the neurons were treated with different concentrations of GCF, cck8 was used to evaluate the cell viability, so as to judge the cytotoxicity of GCF. The phosphorylation level of Akt was used to illustrate the effect of GCF on Whether nerve cells have a protective effect.
[0038] 1. Main experimental reagents and instruments
[0039] GCF was synthesized by our laboratory; Neurobasal, provided by Gibco; B27, provided by Gibco; FBS, provided by Invitrogen; glutamax, provided by Invitrogen; Glutamicacid, provided by sigma; polylysine, provided by sigma. RIPA lysate was provided by Beijing Pulilai Gene Technology Co., Ltd.; BCA kit was provided by American Thermo Company; protein marker was provided by American Thermo Company; glycine was provided by Wuhan Kerui Biotechnology Co., Ltd.; Tris...
Embodiment 3
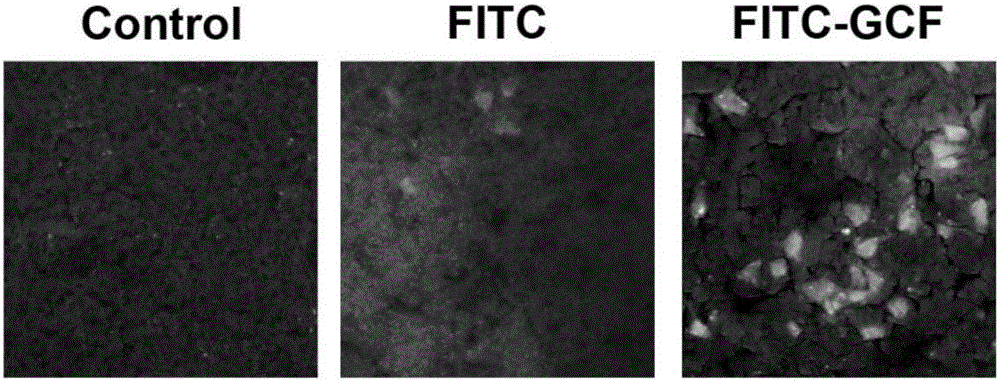
[0059] [Example 3] Establish a rat cerebral ischemia model, inject fluorescently labeled GCF intravenously, and observe the ability of GCF to penetrate the blood-brain barrier as a whole
[0060] 1. The main reagents and instruments of the experiment
[0061] FITC, Wuhan Minghao Biotechnology Co., Ltd.; FITC-GCF, Wuhan Minghao Biotechnology Co., Ltd.; OCT glue, Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.; pathological tissue slicer, Leitz, Germany, type 1512; fluorescence microscope, Ningbo Sunny Instrument Co., Ltd.; Paraformaldehyde, Sinopharm Chemical Reagent Co., Ltd.
[0062] 2. Experimental principle
[0063]FITC (fluorescein isothiocyanate): It is yellow or orange-yellow crystalline powder with a molecular weight of 389.4, a maximum absorption wavelength of 490-495nm, and a maximum emission wavelength of 520-530nm, showing bright yellow-green fluorescence. The broadest fluorescein.
[0064] FITC-GCF: GCF is fluorescently labeled. FITC is combined with GCF tripe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com