Inactivated vaccine prepared through mink pseudomonas aeruginosa strain SD17
An inactivated vaccine, Pseudomonas aeruginosa technology, applied in the direction of veterinary vaccines, vaccines, medical preparations containing active ingredients, etc., can solve the problems of weak immunogenicity, low immune cross protection, etc. Mortality reduction, epidemic and transmission prevention, economic loss reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
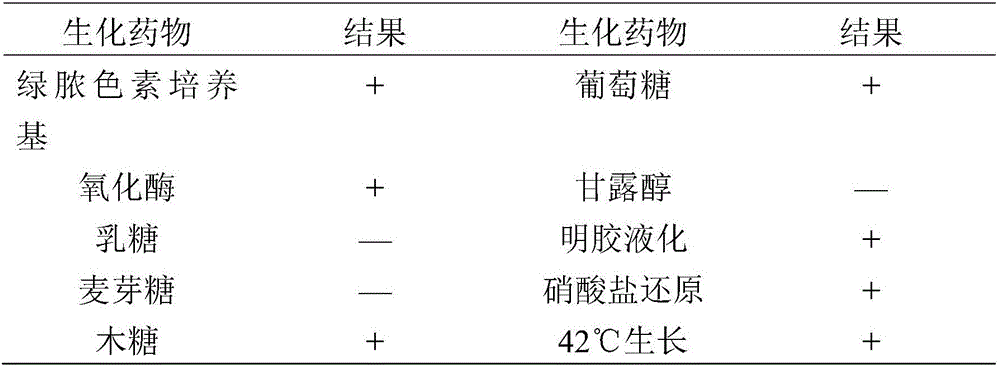
[0013] The Pseudomonas aeruginosa strain G type SD17 was deposited in the China Center for Type Culture Collection (CCTCC) of Wuhan University in Wuhan, China on February 22, 2016, and the preservation number is CCTCC NO: M2016069.
[0014] production medium
[0015] Hexadecanetrimethylammonium bromide agar medium (NAC) preparation: Weigh 4g of NAC powder and add it to 100mL of distilled water, mix thoroughly, sterilize at 121°C for 15min, cool to 50°C to 60°C, pour the plate for later use.
[0016] Preparation of improved nutrient broth medium (NB): Weigh 5g of NB powder into 100mL of distilled water, and sterilize at 121°C for 15min. It is prepared by adding a nutrient broth medium with a final concentration of 2% fetal bovine serum before use, and mixing evenly.
[0017] Fermentation medium preparation: 2% peptone, 0.6% beef extract, 0.5% sodium chloride, 0.5% glucose, made with deionized water. 2% of healthy fetal bovine serum was added.
[0018] 1.1 Isolation and purif...
Embodiment 2
[0047] Application of mink Pseudomonas aeruginosa G type SD17 strain in preparation of inactivated vaccine.
[0048] The mink Pseudomonas aeruginosa G type SD17 strain was taken, activated and inoculated into the medium respectively, the bacterial liquid was collected after cultivation, inactivated by formaldehyde solution, mixed with propolis adjuvant to prepare inactivated propolis vaccine.
[0049] The preferred operation steps are as follows:
[0050] 2.1 Strain selection
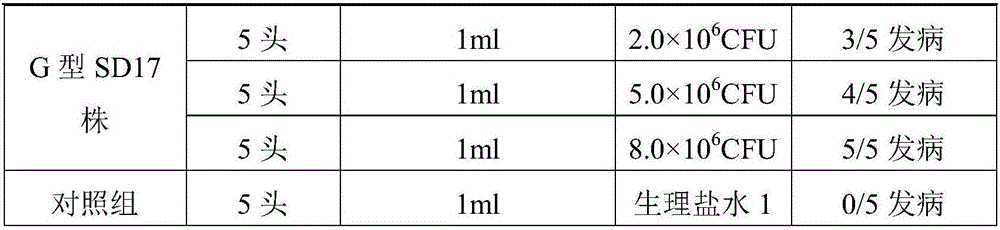
[0051] The strain used for making seedlings was Pseudomonas aeruginosa G type SD17 strain of mink, its colony morphology, cell characteristics, biochemical characteristics, and culture characteristics were all stable, and it had strong pathogenicity to mink. 1.0mL bacterial liquid (8.0×10 6 CFU / mL) injection can cause 100% of 21 to 42 days old mink disease. The immunogenicity of mink Pseudomonas aeruginosa G type SD17 strain is good, and the minimum immune dose should be ≥4.0×10 9 .
[0052] 2.2 Pre...
Embodiment 3
[0092] Application of mink Pseudomonas aeruginosa G type SD17 strain in preparation of inactivated vaccine.
[0093] Take Pseudomonas aeruginosa G type SD17 strain from mink, inoculate the culture medium after activation, collect the bacterial liquid after culture, inactivate with formaldehyde solution, and mix with water adjuvant to prepare inactivated vaccine.
[0094] The preferred operation steps are as follows:
[0095] 2.1 Strain selection
[0096] The strain used for making seedlings was Pseudomonas aeruginosa G type SD17 strain of mink, its colony morphology, cell characteristics, biochemical characteristics, and culture characteristics were all stable, and it had strong pathogenicity to mink. 1.0mL bacterial liquid (8.0×106 CFU / mL) injection can cause 100% of 21 to 42 days old mink disease. The immunogenicity of mink Pseudomonas aeruginosa G type SD17 strain is good, and the minimum immune dose should be ≥4.0×10 9 .
[0097] 2.2 Preparation of strains for productio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com