Research and application of a novel sustained release preparation adjuvant
A release excipient, a new type of technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0043] Example 1: Preparation of succinylated WPI whey protein isolate excipient
[0044] Maintain the temperature at 35°C-65°C, dissolve 100g of WPI whey protein isolate in 800ml of distilled water, keep the pH of the solution between 7.0-11.0 during the dissolution process, and add the corresponding anhydride, continue to keep the pH value of the solution between 7.0-11.0, measure the degree of protein acylation by methods such as o-phthalaldehyde method or ninhydrin method, and obtain the protein with the target degree of acylation, and the pH value is finally Stabilize between 8.0-8.5 for more than 30 minutes to terminate the reaction. Dialyze the acylated protein solution at 4°C for 24 hours with a dialysis bag with a molecular cut-off of 1000DA, and then dry it.
[0045] The preparation process of other auxiliary materials such as acetylated or succinylated whey protein WPC and soybean protein is the same as that shown in Example 1.
Embodiment 2
[0046] Example 2: Preparation of WPI protein excipient diclofenac sodium sustained-release tablets with different degrees of succinylation (taking 0.4g / tablet as an example)
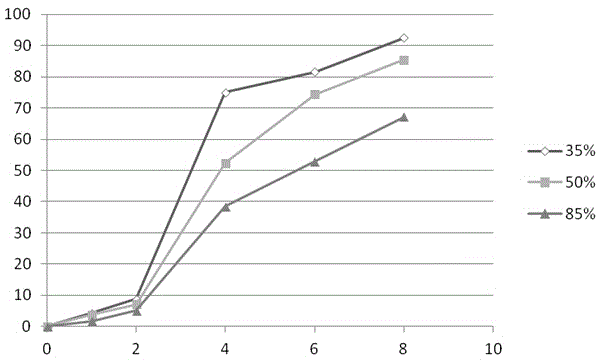
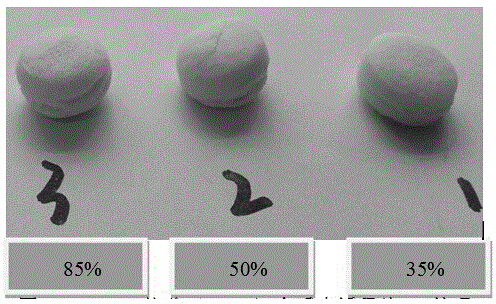
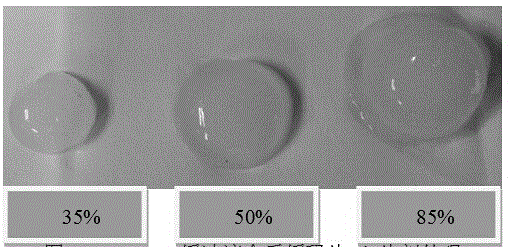
[0047] According to the method shown in Example 1, WPI whey protein isolate adjuvant materials with acylation degrees of 35%, 50%, and 85% were prepared respectively.
[0048] Precisely weigh 40g of diclofenac sodium (taking 10% drug loading as an example), add the prescribed amount of microcrystalline cellulose (5%), precisely weigh the prescribed amount of WPI acylated protein excipients, and mix the powder evenly and use Shanghai Tianfan Instruments to manufacture Diclofenac sodium sustained-release tablets were prepared by direct compression on a tablet press at room temperature.
[0049] 1) Simulate the gastrointestinal tract environment with different pH, according to the requirements of the appendix of "Chinese Pharmacopoeia" 2010 edition, investigate the release of this example in the gastric jui...
Embodiment 3
[0054] Example 3: Preparation of sustained-release tablets of allicin derivatives with different succinylation degrees WPI excipients
[0055] According to the method shown in Example 1, WPI whey protein isolate protein supplements with acylation degrees of 0%, 35%, 40%, 55%, 70%, 85%, and 100% were prepared respectively.
[0056] Precisely weigh 40g of allicin derivatives (10% drug loading as an example), add the prescribed amount of micropowder silica gel (0.5%), precisely weigh the prescribed amount of WPI acylated protein excipients, mix the powder evenly, and use Shanghai Tianfan Pharmaceutical Machinery to manufacture Sustained-release tablets were prepared by direct compression on the tablet press of the factory at room temperature.
[0057] 4) Simulate the gastrointestinal tract environment with different pH, according to the requirements of the appendix of "Chinese Pharmacopoeia" 2010 edition, investigate the release of this example in the gastric juice environment, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com