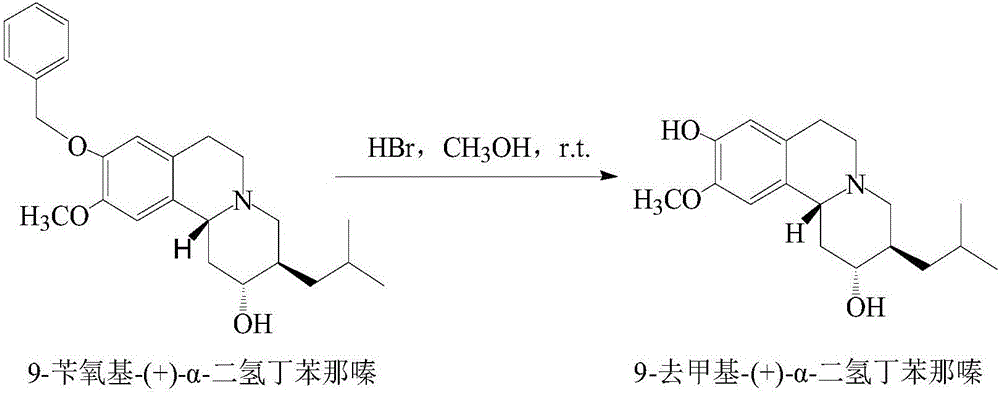
Synthesis method of 9-demethyl-(+)-alpha-dihydrotetrabenazine
A technology of dihydrotetrabenazine and a synthetic method, which is applied in the direction of organic chemistry, can solve the problems of unfavorable large-scale industrial production, complex reaction operation, harsh reaction conditions, etc., and achieve short reaction time, simple reaction operation and low preparation cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The synthetic method of present embodiment 9-demethyl-(+)-α-dihydrotetrabenazine comprises the following steps:
[0044] Dissolve 1mmol of 9-benzyloxy-(+)-α-dihydrotetrabenazine in 5mL of methanol, add 2mmol of hydrobromic acid dropwise under stirring, and react at room temperature for 0.5h to obtain 9-desmethyl-(+ )-alpha-dihydrotetrabenazine crude product reaction solution;
[0045] It also includes the following steps of separation and purification treatment:
[0046](1) concentrating the reaction solution of the crude 9-desmethyl-(+)-α-dihydrotetrabenazine under reduced pressure to obtain a solid;
[0047] (2) Dissolving the solid in a mixed solvent of dichloromethane / ethyl acetate / methanol (volume ratio 1:1:0.05), standing still and crystallizing to obtain crystals;
[0048] (3) Dissolve the crystals in water, adjust the pH value to 7-9 with aqueous sodium hydroxide solution, extract with ether, and concentrate the organic phase under reduced pressure to obtain 9...
Embodiment 2
[0054] The synthetic method of present embodiment 9-demethyl-(+)-α-dihydrotetrabenazine comprises the following steps:
[0055] Dissolve 1mmol of 9-benzyloxy-(+)-α-dihydrotetrabenazine in 5mL of ethanol, add 5mmol of hydrobromic acid dropwise under stirring, and react for 1h at room temperature to obtain 9-desmethyl-(+) -The reaction liquid of α-dihydrotetrabenazine crude product;
[0056] It also includes the following steps of separation and purification treatment:
[0057] (1) concentrating the reaction solution of the crude 9-desmethyl-(+)-α-dihydrotetrabenazine under reduced pressure to obtain a solid;
[0058] (2) Dissolving the solid in a mixed solvent of dichloromethane / ethyl acetate / methanol (volume ratio 0.8:1.2:0.04), standing still and crystallizing to obtain crystals;
[0059] (3) Dissolve the crystals in water, adjust the pH value to 7-9 with potassium hydroxide aqueous solution, extract with ether, and concentrate the organic phase under reduced pressure to ob...
Embodiment 3
[0065] The synthetic method of present embodiment 9-demethyl-(+)-α-dihydrotetrabenazine comprises the following steps:
[0066] Dissolve 1mmol of 9-benzyloxy-(+)-α-dihydrotetrabenazine in 5mL of methanol, add 20mmol of hydrobromic acid dropwise under stirring, and react at room temperature for 1.5h to obtain 9-desmethyl-(+ )-alpha-dihydrotetrabenazine crude product reaction solution;
[0067] It also includes the following steps of separation and purification treatment:
[0068] (1) concentrating the reaction solution of the crude 9-desmethyl-(+)-α-dihydrotetrabenazine under reduced pressure to obtain a solid;
[0069] (2) Dissolving the solid in a mixed solvent of dichloromethane / ethyl acetate / methanol (volume ratio 1.2:0.8:0.06), standing still and crystallizing to obtain crystals;
[0070] (3) Dissolve the crystals in water, adjust the pH value to 7-9 with aqueous sodium carbonate solution, extract with ether, and concentrate the organic phase under reduced pressure to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com