Single-ion polymer electrolyte and preparation method thereof and lithium-ion secondary battery
A secondary battery and polymer technology, applied in secondary batteries, circuits, electrical components, etc., can solve the problem of low conductivity, achieve the effect of improving conductivity, high charge transmission capacity, and improving electrical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] In addition, the present invention also provides a method for preparing a single-ion polymer electrolyte, which includes the following steps: combining a π-conjugated compound containing multiple hydroxyl groups with sp 3 The small-molecule lithium salt of hybridized boron undergoes condensation polymerization to form a single-ion polymer electrolyte.
[0072] Here "contains sp 3 Small molecule lithium salt of hybrid boron", "sp 3 Hybridized boron" is sp 3 Boron atoms formed by hybridization. "Small molecule lithium salt" is a relative concept, usually referring to lithium salts with molecular weight less than 500. The "π-conjugated compound containing a plurality of hydroxyl groups" herein means a π-conjugated compound containing two or more hydroxyl groups. And "π-conjugated compounds" refer to compounds containing delocalized large π-bonds, such as p-xylylenedimethanol and the like.
[0073] In the above method provided by the present invention, as long as the π...
Embodiment 1
[0104] (1) Preparation of single-ion polymer electrolyte by polycondensation of 2,5-dihydroxyterephthalic acid and lithium tetramethoxyboron
[0105] 2,5-Dihydroxyterephthalic acid was reacted with an excess of hexamethyldisilazane in anhydrous 1,2-dichloroethane. The reaction solution obtained by the reaction was distilled off the solvent under reduced pressure to obtain an intermediate (silylated product).
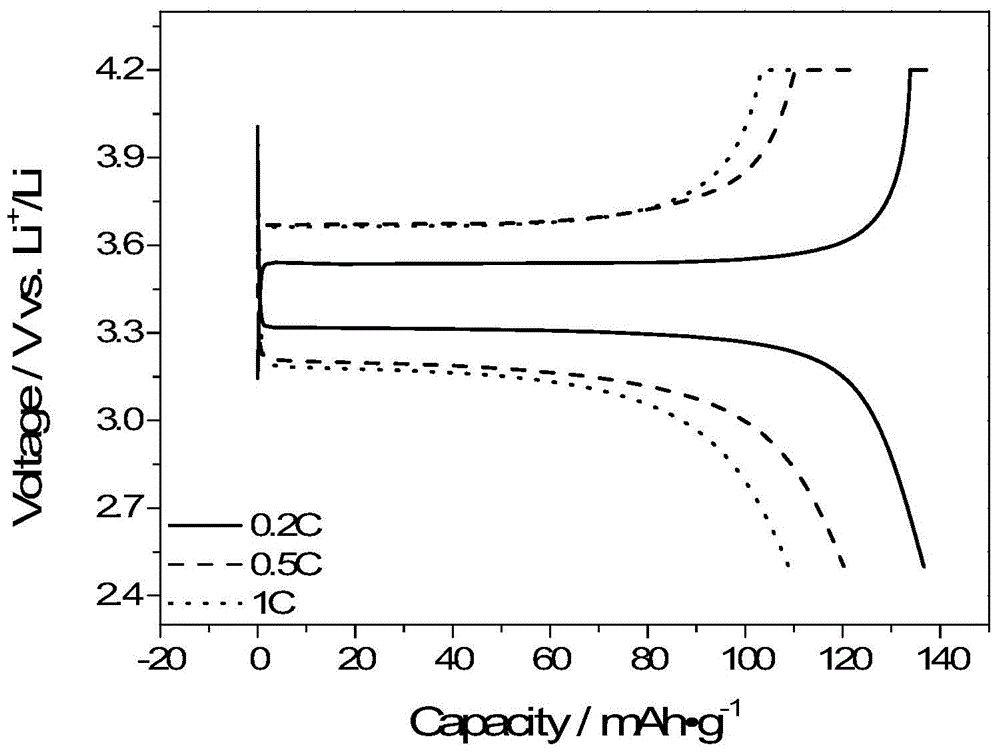
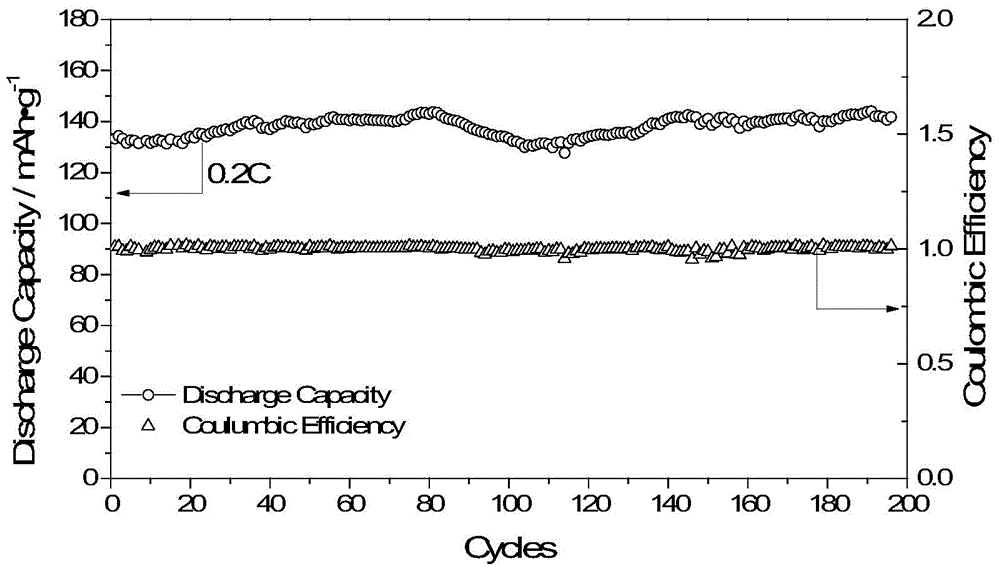
[0106] Dissolve 1.217g (2.5mmol) of the above silylated product, 0.3547g (2.5mmol) lithium tetrasilylmethoxyborate in 20mL of anhydrous tetrahydrofuran, react at 45°C for three days, and filter the reaction solution to obtain a white precipitation. Washed several times with anhydrous tetrahydrofuran, dried under vacuum at 60°C for 48 hours to obtain a single-ion polymer electrolyte, named PLBDB. Boron spectrum nuclear magnetic detection shows that the boron in this polymer is sp3 hybridization, and the peak position is 3.83 (BF 3 ·Et 2 O as a reference). GPC test it...
Embodiment 2
[0116] (1) Preparation of single-ion polymer electrolyte by polycondensation of terephthalmic dimethanol and lithium borohydride
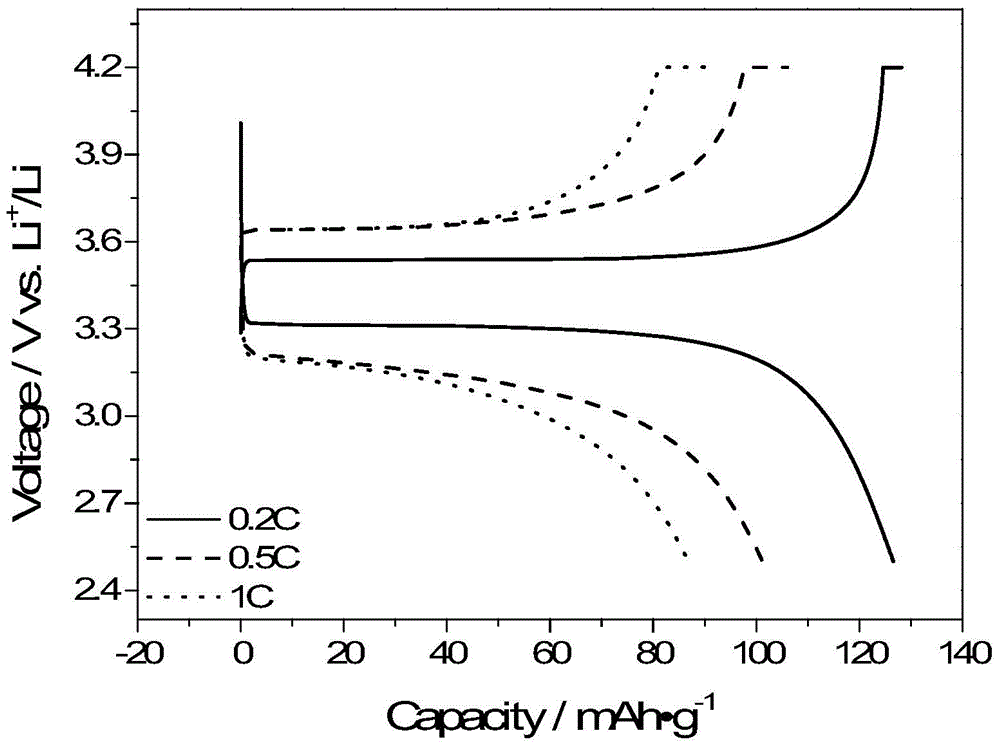
[0117] Dissolve 1.382g (10mmol) of terephthalic acid and 0.1089g (5mmol) of lithium borohydride in 20mL of anhydrous tetrahydrofuran. After reacting at 45°C for three days, the reaction solution was filtered to obtain a white precipitate. After washing several times with anhydrous tetrahydrofuran and drying in vacuum at 60°C for 48 hours, a single-ion polymer electrolyte was obtained, which was named PLBPB. Boron NMR detection shows that the boron in the polymer is sp3 hybridization, and the peak position is 3.09 (BF 3 ·Et 2 O as a reference). GPC test its weight average molecular weight is 11768, number average molecular weight is 11500. Its decomposition temperature is 400°C in thermogravimetric test.
[0118] (2) Using the above-mentioned single-ion polymer electrolyte as a raw material to prepare the electrolyte membrane of the battery
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com