Method for preparing difluoro-lithium phosphate and lithium-ion battery non-aqueous electrolyte
A non-aqueous electrolyte and lithium difluorophosphate technology, which is applied in secondary batteries, chemical instruments and methods, circuits, etc., can solve the difficulties in the preparation of difluorophosphate, the difficulty in the synthesis of lithium difluorophosphate, and the lack of effective methods, etc. problems, achieve good electrochemical performance, reduce production costs, and simplify the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
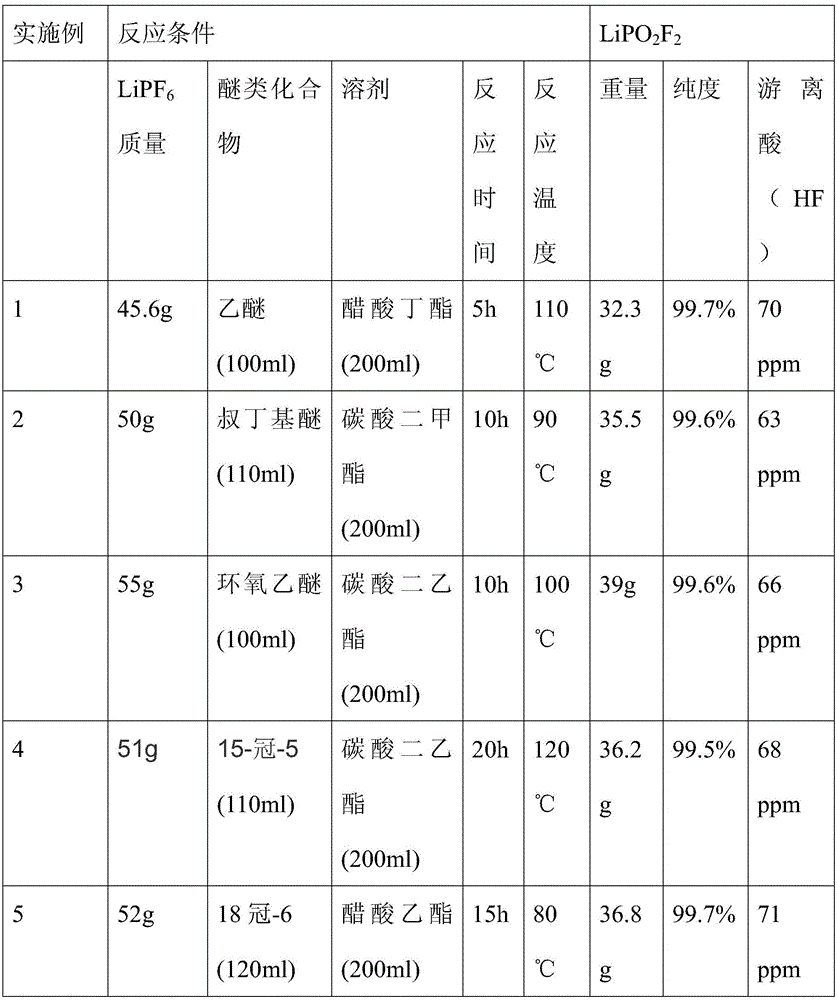
[0027] A preparation method of lithium difluorophosphate of the present invention comprises the following steps: under the protection of an inert gas, preferably, the inert gas is one of nitrogen or argon, and an organic solvent is used as the reaction medium, preferably, the organic solvent is Carbonate solvents or carboxylate solvents, the carbonate solvents or the carboxylate solvents include dimethyl carbonate, diethyl carbonate, butyl acetate, ethyl acetate, lithium hexafluorophosphate and ether compounds React in a reaction vessel with a PFA or PTFE protective layer on the inner layer. Preferably, the ether compound is a chain ether compound or a cyclic ether compound. The chain ether compound is such as ether, tert-butyl ether, etc., and the cyclic Ether compounds such as 18 crown-6, epoxy ether, the volume ratio of the moles of lithium hexafluorophosphate to the solvent is greater than 1.5mol / L, and the ratio of the moles of ether bonds (C-O-C) to the moles of lithium h...
Embodiment 1~5
[0032] Under the protection of a nitrogen atmosphere, firstly add ether compounds into the reactor, then add an organic solution containing a certain concentration of lithium hexafluorophosphate, and control the reaction temperature, time and stirring rate. The reaction conditions are shown in Table 1.
[0033] The above reaction liquid is processed, and the product is obtained by suction filtration, washing and drying. The reaction conditions and analysis results are shown in Table 1.
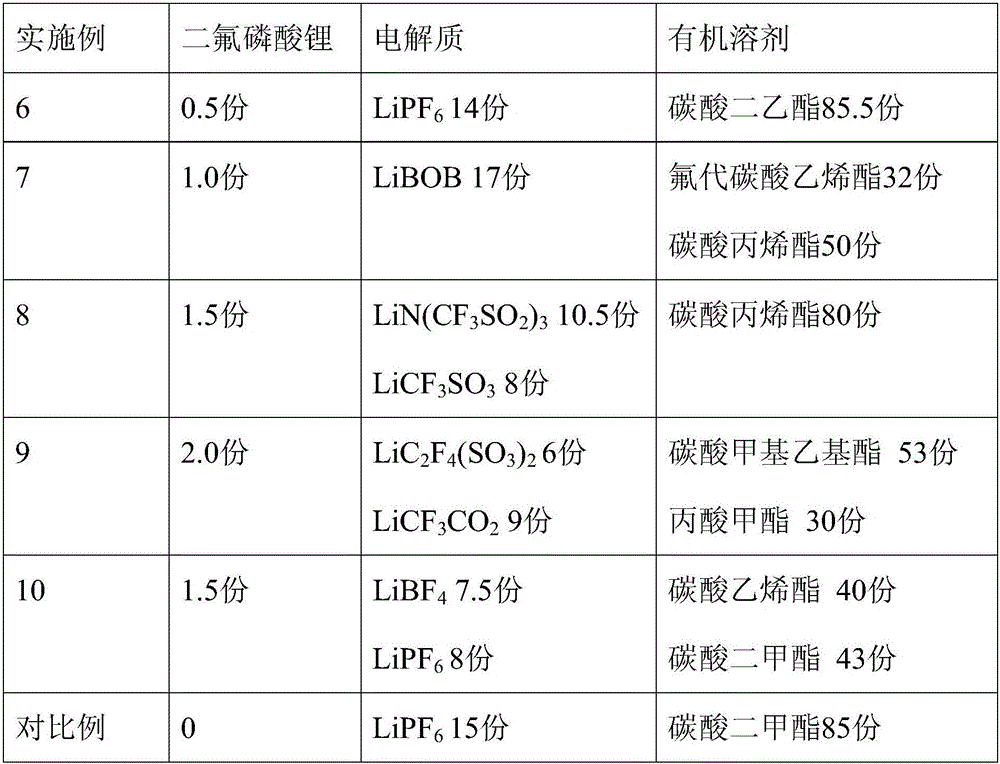
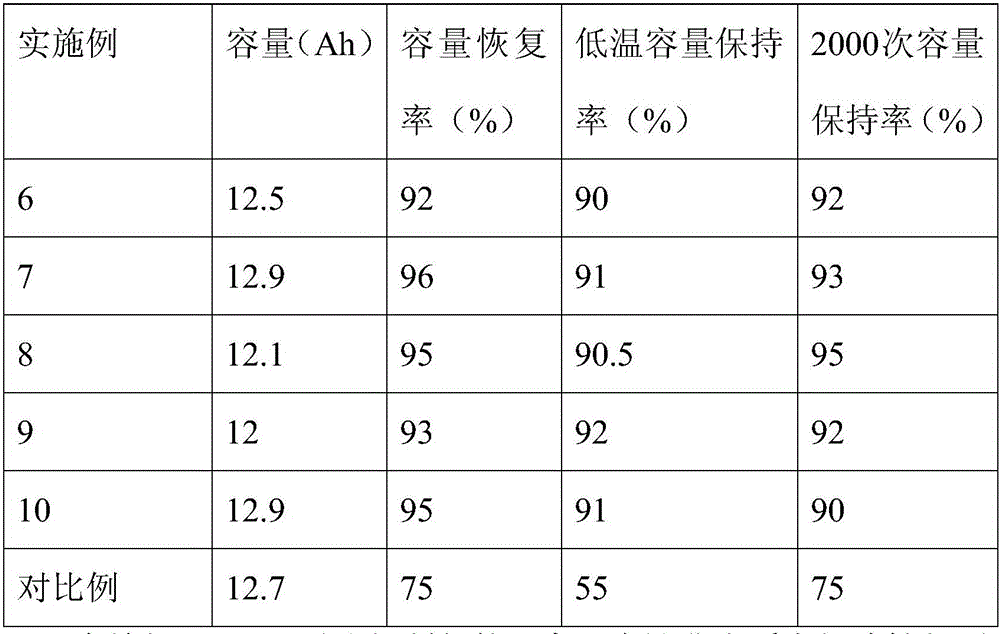
Embodiment 6~10
[0034] Embodiment 6~10 and comparative example
[0035] The formula of the lithium ion battery non-aqueous electrolyte of lithium difluorophosphate is shown in Table 2, and its preparation method is as follows:
[0036] In a glove box under the protection of argon, the electrolyte salt is added to the organic solvent, mixed evenly, and then the lithium difluorophosphate prepared in any one of Examples 1 to 5 is added to obtain a non-aqueous electrolytic solution for a lithium-ion battery. liquid.
[0037] The formulation of the non-aqueous electrolyte for lithium-ion batteries is shown in Table 2.
[0038] Preparation of positive electrode
[0039] The positive electrode is prepared through the following steps: 90% by weight of lithium iron phosphate, 5% by weight of polyvinylidene fluoride (PVdF), and 5% by weight of acetylene black are mixed; N-methylpyrrolidone is added to form a slurry; the slurry Coated on both surfaces of an aluminum current collector, followed by dry...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com