S-2-hydroxy-3-methoxy-3, 3-dibenzylpropionic acid and preparation method thereof
A technology of diphenylpropionic acid and diphenylpropionate, which is applied in the field of drug synthesis, can solve problems such as low yield, high product cost, and long construction period, and achieve cost reduction, short synthesis cycle, simple and feasible operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] (1) Sodium 2-hydroxy-3-methoxy-3,3-diphenylpropionate
[0063] Dissolve 230g of diphenyl-2,3-epoxypropionate in 600mL of methanol, stir and dissolve at room temperature, add 5.7g of p-toluenesulfonic acid, stir at 20-25°C for 0.5h, heat to reflux, and then drop into 10% sodium hydroxide 800mL, reflux and stir for 1.5h after the dropwise addition, add 90ml of water after concentrating methanol, place in ice bath and stir for 0.5h, then filter, wash the solid with cold water, and blow dry at 60°C.
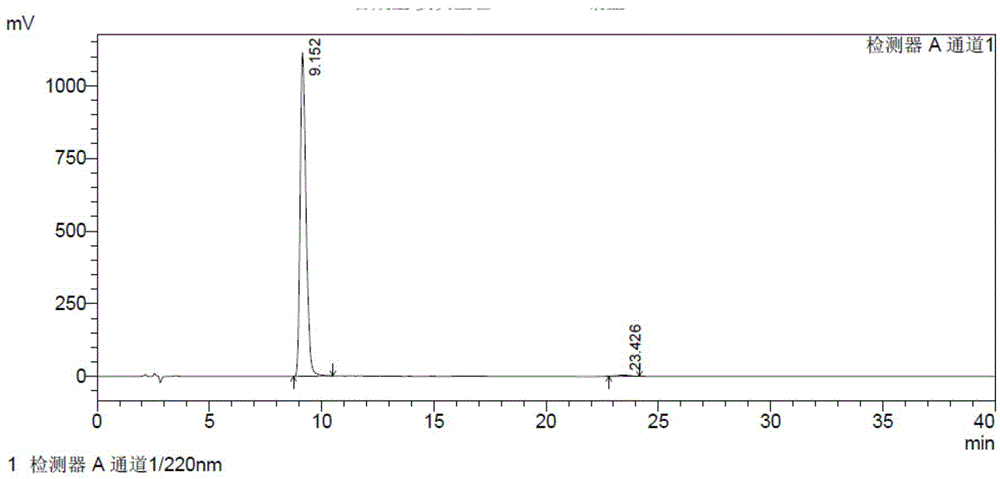
[0064] The resulting solid was mixed with 60 ml of ethanol and 30 ml of petroleum ether and stirred for 1 h, filtered, and dried under vacuum at room temperature to obtain about 160 g of a white solid. The HPLC test result of 2-hydroxy-3-methoxy-3,3-diphenylpropionate sodium is shown in Table 1 and figure 1 .
[0065] (2) S-2-Hydroxy-3-methoxy-3,3-diphenylpropionic acid
[0066] Add 100g of synthesized sodium 2-hydroxy-3-methoxy-3,3-diphenylpropionate into methyl tert-butyl...
Embodiment 2
[0074] (1) Potassium 2-hydroxy-3-methoxy-3,3-diphenylpropionate
[0075] Dissolve 210g of diphenyl-2,3-epoxypropionate in 600mL of methanol, stir and dissolve at room temperature, add 15mL of 10% sulfuric acid, stir at 20-25°C for 1h, heat to reflux, and then drop in 10% hydrogen Potassium oxide solution 1100ml, reflux and stir for 1.5h after the dropwise addition, add 400ml of water after concentrating methanol, put in ice bath and stir for 0.5h, then filter, wash the solid with cold water, and blow dry at 60°C.
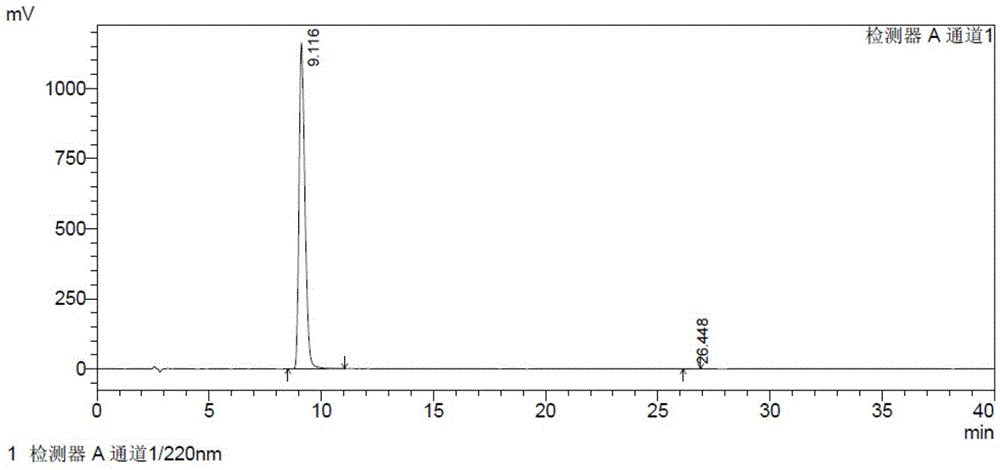
[0076] The resulting solid was stirred with 130 ml of ethyl acetate for 1 h, filtered, and dried under vacuum at room temperature to obtain about 143 g of a white solid. The HPLC test result of 2-hydroxy-3-methoxy-3,3-diphenylpropionate potassium is shown in Table 4 and Figure 4 .
[0077] (2) S-2-Hydroxy-3-methoxy-3,3-diphenylpropionic acid
[0078] Add 105g of synthesized potassium 2-hydroxy-3-methoxy-3,3-diphenylpropionate into methyl tert-butyl ether (MTB, 2...
Embodiment 3
[0086] (1) Lithium 2-hydroxy-3-methoxy-3,3-diphenylpropionate
[0087] Dissolve 230g of diphenyl-2,3-epoxypropionate in 600mL of methanol, stir and dissolve at room temperature, add 5.5g of benzenesulfonic acid, stir at 20-25°C for 0.5h, heat to reflux, and then drop into 10 %Lithium hydroxide 500mL, reflux and stir for 1.5h after the dropwise addition, add 600ml of water after concentrating the methanol, stir in an ice bath for 0.5h, filter, wash the solid with cold water, and blow dry at 60°C.
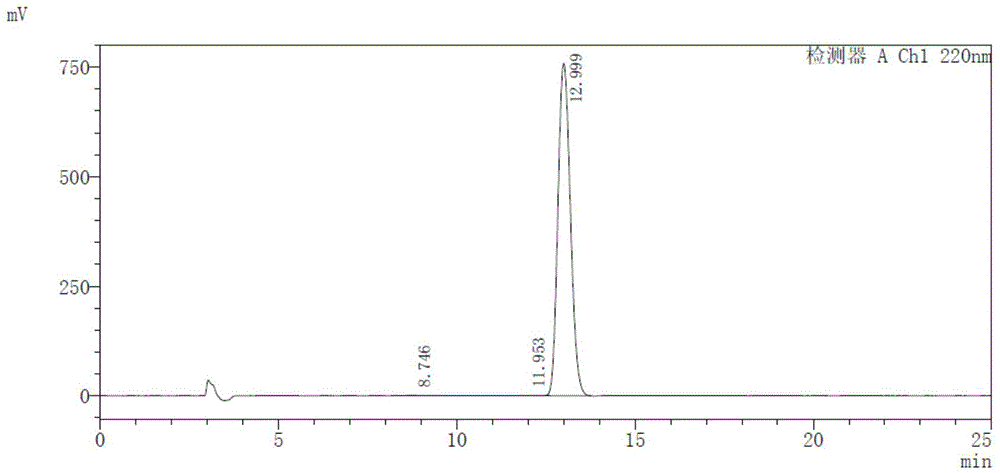
[0088] The resulting solid was mixed with 150 ml of dichloromethane and 50 ml of n-hexane and stirred for 1 h, filtered, and dried under vacuum at room temperature to obtain about 156 g of a white solid. The HPLC test result of 2-hydroxy-3-methoxy-3,3-diphenylpropionate lithium is shown in Table 7 and Figure 7 .
[0089] (2) S-2-Hydroxy-3-methoxy-3,3-diphenylpropionic acid
[0090] Add 95g of synthesized lithium 2-hydroxy-3-methoxy-3,3-diphenylpropionate into methyl tert-butyl et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com