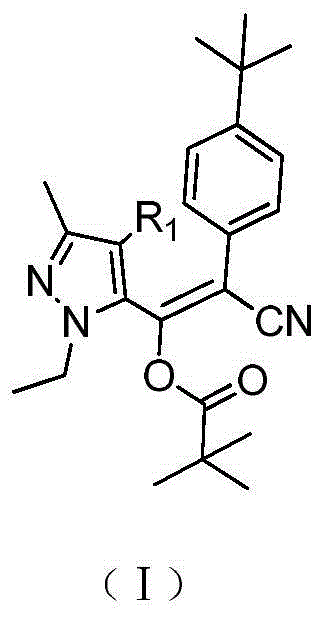
Preparation method of pyrazolyl acrylonitrile compound
A technology of pyrazolyl acrylonitrile and compound, which is applied in the field of preparation of azolyl acrylonitrile compounds, can solve the problems of complex post-processing, large amount of three wastes, and low yield of intermediate synthesis, and achieve good dispersibility and total yield. The effect of improving the efficiency and reducing the discharge of "three wastes"
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Z-2-(4-(tert-butyl)phenyl)-2-cyano-1-(1-ethyl-3-methyl-1hydro-5-pyrazolyl)vinyl pivalate (Table Synthesis of compound 1) in 1
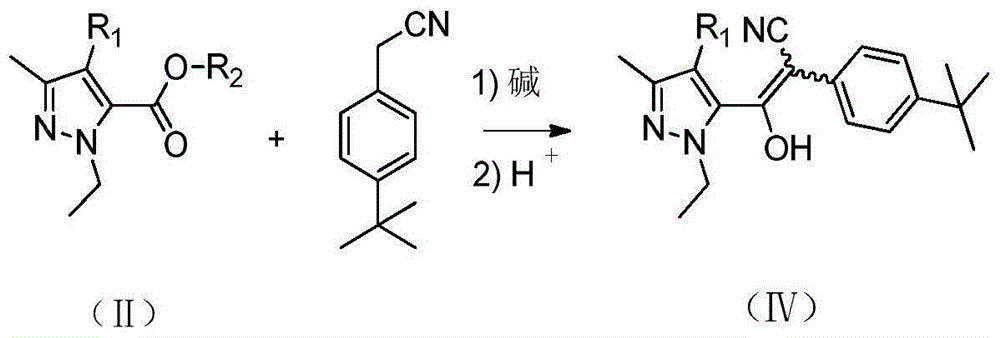
[0032] Add 43.7g (0.8mol) of sodium methoxide, 140g (0.8mol) of p-tert-butylphenylacetonitrile and 900g of toluene into a reaction flask equipped with a rectification tower, and heat up to 60°C. A solution of 170 g (1.0 mol) of methyl 1-ethyl-3-methyl-1-hydrogen-5-pyrazolecarboxylate and 300 g of toluene was slowly added dropwise, and the dropwise was completed in about 1 hour. After the dropwise addition, the temperature was raised to reflux. During the reaction, the low boilers with a boiling point lower than 65°C were continuously separated from the top of the tower and reacted for 5 hours to obtain 2-(4-(tert-butyl)phenyl)-1-(- 1-ethyl-3-methyl-1hydro-5-pyrazolyl)-2-cyanovinyl alcohol sodium salt suspension in toluene.
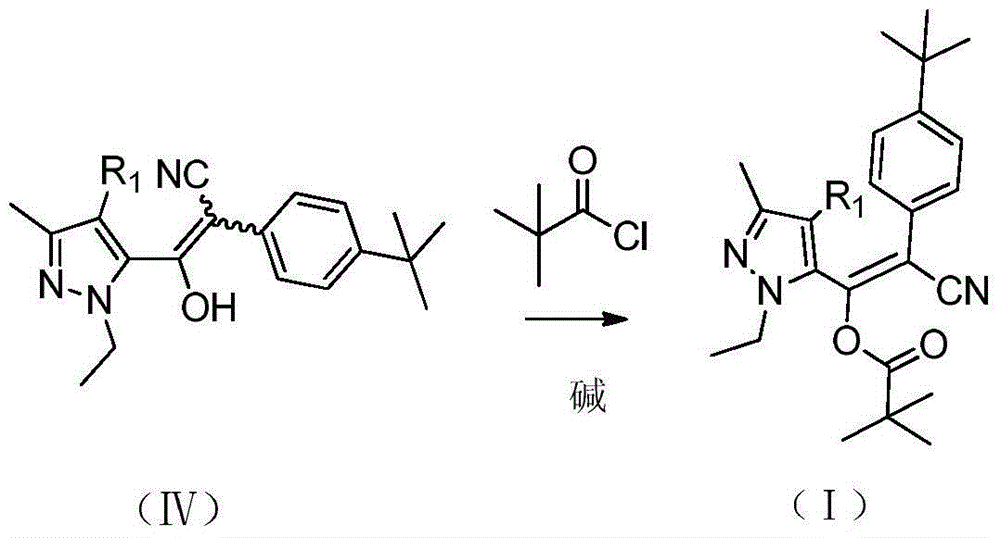
[0033] At 80-90° C., 98 g (0.8 mol) of pivaloyl chloride was added dropwise to the reaction mixture in the previous ste...
Embodiment 2
[0037] Z-2-(4-(tert-butyl)phenyl)-2-cyano-1-(1-ethyl-3-methyl-1hydro-5-pyrazolyl)vinyl pivalate (Table Synthesis of compound 1) in 1
[0038] In a reactor equipped with a rectification tower, 11Kg (20.2mol) of sodium methylate and 180Kg of toluene were added, and the temperature was raised to 100°C. Slowly add a solution of 33Kg (189mol) p-tert-butylphenylacetonitrile, 30Kg (177mol) 1-ethyl-3-methyl-1-hydrogen-5-pyrazolecarboxylic acid methyl ester and 50Kg toluene for about 1.5 hours. Finish. After the dropwise addition, the temperature was raised to reflux. During the reaction, the low boilers with a boiling point lower than 65°C were continuously separated from the top of the tower, and reacted for 6 hours to obtain 2-(4-(tert-butyl)phenyl)-1-(- 1-ethyl-3-methyl-1hydro-5-pyrazolyl)-2-cyanovinyl alcohol sodium salt suspension in toluene.
[0039] At 100-105° C., 24 Kg (197 mol) of pivaloyl chloride was added dropwise to the reaction mixture in the previous step, and the a...
Embodiment 3
[0041]Z-2-(4-(tert-butyl)phenyl)-2-cyano-1-(1-ethyl-3-methyl-4-chloro-1hydro-5-pyrazolyl)pivalic acid Synthesis of Vinyl Esters (Compound 5 in Table 1)
[0042] Add 54.6g (1.0mol) of sodium methoxide and 900g of toluene into the reaction flask equipped with a rectification tower, and raise the temperature to 80°C. Slowly add dropwise a solution of 205g (1.0mol) 4-chloro-1-ethyl-3-methyl-1hydrogen-5-pyrazolecarboxylic acid methyl ester, 175g (1.0mol) p-tert-butylphenylacetonitrile and 300ml of toluene , It takes about 2 hours to drip. After the dropwise addition, the temperature was raised to reflux. During the reaction, the low boilers with a boiling point lower than 65°C were continuously separated from the top of the tower, and reacted for 3 hours to obtain 2-(4-(tert-butyl)phenyl)-1-(4 - Chloro-1-ethyl-3-methyl-1hydro-5-pyrazolyl)-2-cyanovinyl alcohol sodium salt suspension in toluene.
[0043] At 105-110° C., 122 g (1.0 mol) of pivaloyl chloride was added dropwise to th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com