Preparation method for carbonic acid linalool oxide (pyran type and furan type) ester ethyl ester
A technology of linalool carbonate and oxide, which is applied in the field of preparation of ethyl linalool carbonate oxide, and achieves the effects of low production cost, convenient operation and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
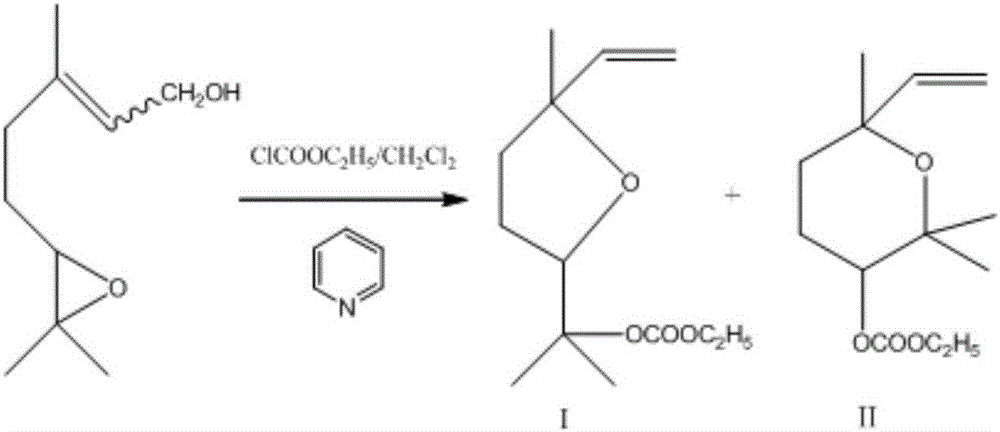
[0021] The preparation method of linalool carbonate (pyran type and furan type) ethyl ester includes the following steps:
[0022] 1) In an ice-water bath, stir a solution consisting of 3,7-dimethyl-6,7-epoxy-2-octene-1-ol, dichloromethane and pyridine until uniform to obtain solution A, 10min Add the dichloromethane solution of ethyl chloroformate dropwise to the solution A while stirring. After the addition, continue to stir and react at room temperature for 10-20 hours. After the reaction is completed, the obtained reaction solution is hydrochloric acid with a concentration of 5% by mass. The aqueous solution adjusts the pH of the reaction solution to neutral to obtain a neutral reaction solution; among them, 3,7-dimethyl-6,7-epoxy-2-octene-1-ol, dichloromethane and pyridine are based on the amount of substance And volume ratio calculation, that is 3,7-dimethyl-6,7-epoxy-2-octene-1-ol: dichloromethane: pyridine is 1mol: 1.20~3.20L: 0.16~0.81L; chloroformic acid The concentrat...
Embodiment 1
[0034] A preparation method of linalool carbonate (pyran type and furan type) ethyl ester comprises the following steps:
[0035] (1) Place a mixed solution consisting of 2.11 grams (98.90%, 12.28mmol) 3,7-dimethyl-6,7-epoxy-2-octene-1-ol, 30ml dichloromethane and 8ml pyridine in In a three-necked flask, add dropwise 2.68 g of ethyl chloroformate in 10 ml of dichloromethane to the solution within 10 minutes at 0-5°C while stirring. After the addition, continue to stir and react at room temperature for 18 hours. The reaction is obtained after the reaction is complete Adjust the pH of the reaction solution to neutral with a hydrochloric acid aqueous solution with a concentration of 5% by mass;
[0036] (2) The reaction solution washed to neutral pH in step (1) was extracted with ether, and the resulting organic layer was washed with anhydrous MgSO 4 After drying and filtering with filter paper the next day, the filtrate obtained was evaporated and concentrated by a rotary evaporator ...
Embodiment 2
[0044] A preparation method of linalool carbonate (pyran type and furan type) ethyl ester comprises the following steps:
[0045] (1) Put 2.02 grams (98.90%, 11.75mmol) 3,7-dimethyl-6,7-epoxy-2-octene-1-ol, 30ml dichloromethane and 10ml pyridine in a mixed solution In a three-necked flask, add 2.55 g of ethyl chloroformate in 10 ml of dichloromethane to the solution in 10 minutes at 0-5°C while stirring. After the addition, continue to stir and react at room temperature for 18 hours. The reaction is obtained after the reaction is complete Adjust the pH of the reaction solution to neutral with a hydrochloric acid aqueous solution with a concentration of 5% by mass;
[0046] (2) The reaction solution washed to neutral pH in step (1) was extracted with ether, and the resulting organic layer was washed with anhydrous MgSO 4 After drying and filtering with filter paper the next day, the filtrate obtained was evaporated and concentrated by a rotary evaporator to obtain 2.44g crude produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com