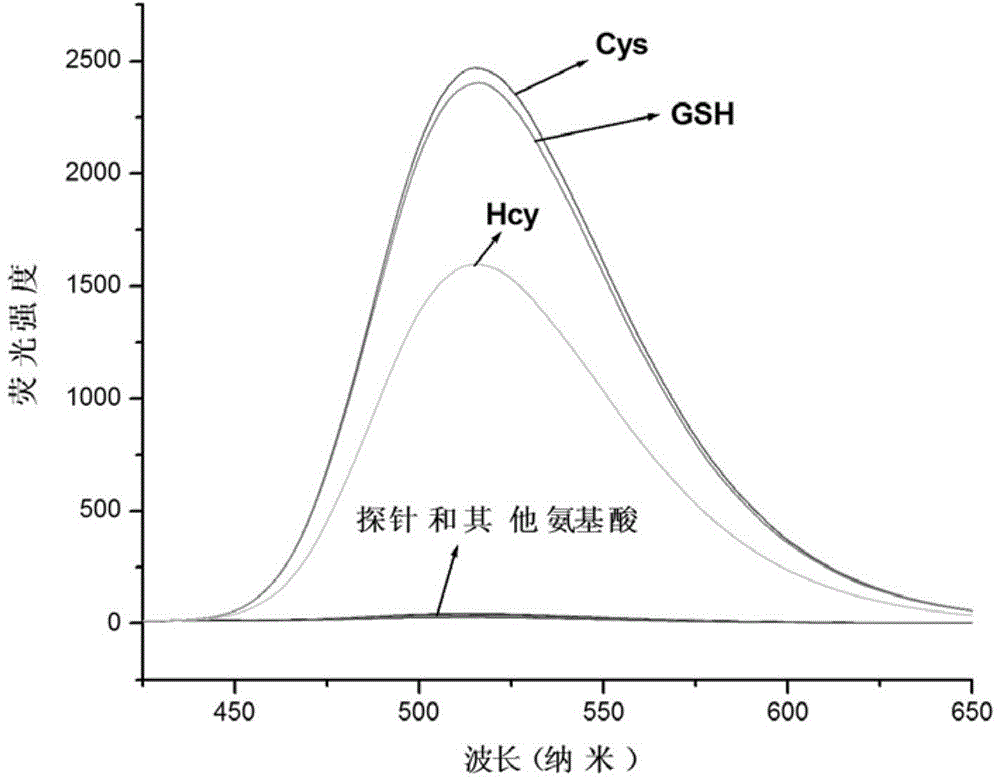
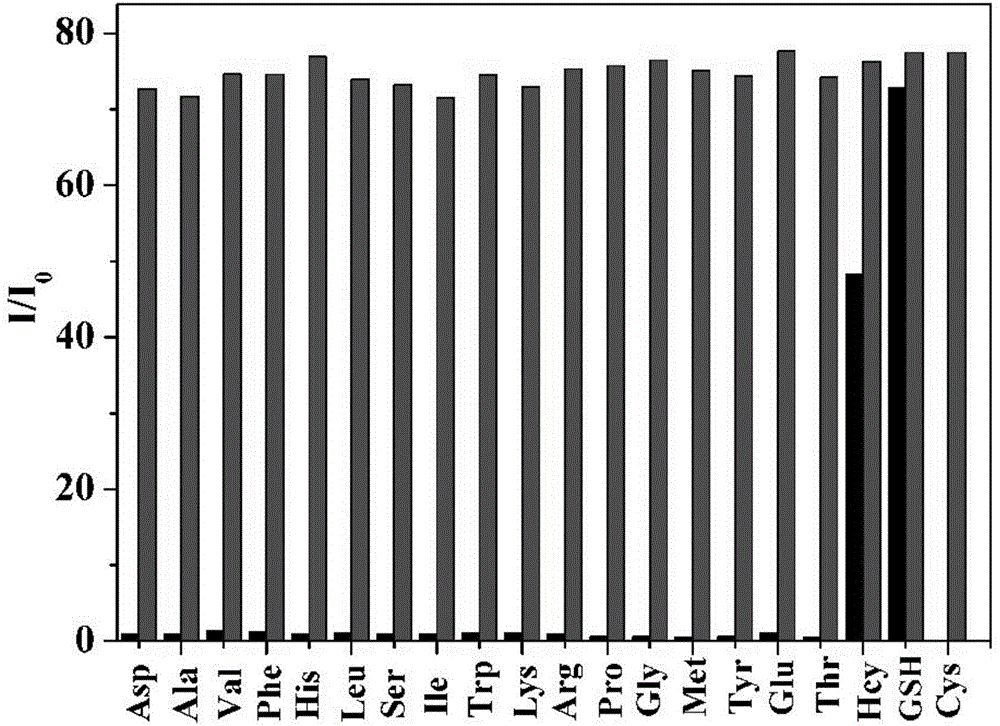
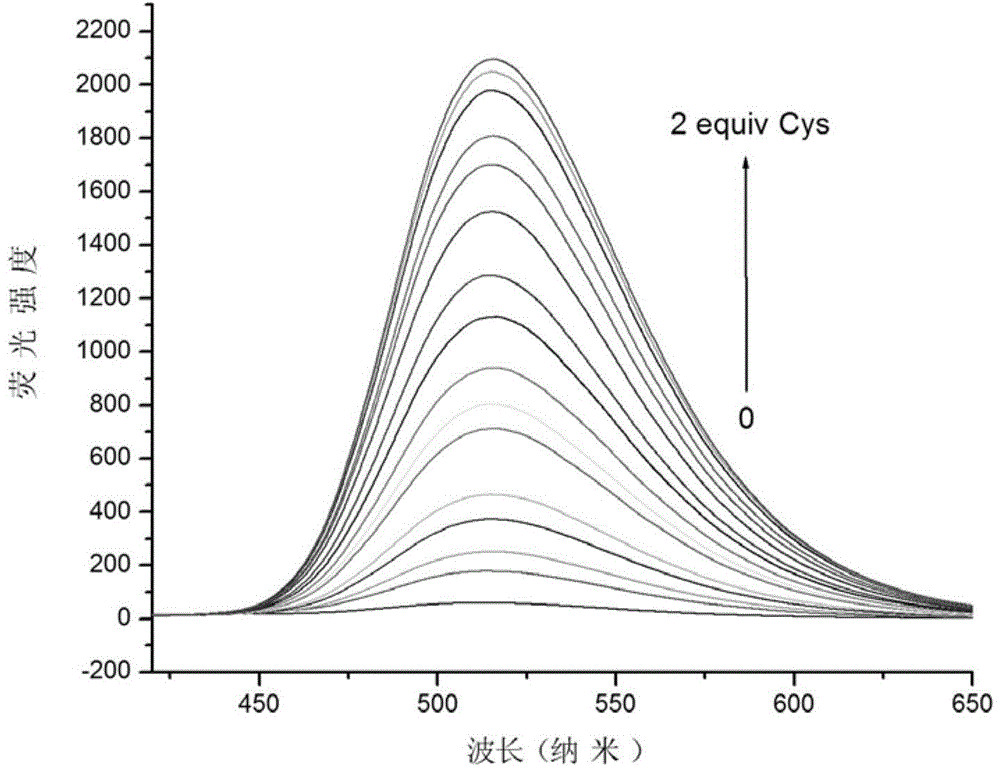
Preparation method and use of mercapto-containing amino acid fluorescent probe
A technology of fluorescent probes and probe molecules, which is applied in the field of chemical analysis and detection, can solve problems such as poor water solubility, and achieve low cost, good selectivity, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of compound 1
[0023] 3-Nitrophthalic anhydride (1.9361g, 10mmol) was dissolved in 20ml of acetic acid, n-butylamine (1.0951g, 15mmol) was slowly added, stirred at room temperature for 10min, heated up, and refluxed at 120°C for 2.5h. After the reaction, cool to room temperature, pour the reaction solution into 50ml of cold water, and after the solid is completely precipitated, filter under reduced pressure, and wash the filter cake with cold water three times (9ml×3) to obtain compound 1 as a white solid. Yield: 2.1252 g. Yield: 85.7%. Compound 1 was characterized as follows: 1HNMR (500MHz, DMSO): δH8.27(d, J=8.1Hz, 1H), 8.16(d, J=7.5Hz, 1H), 8.05(t, J=7.8Hz, 1H), 3.57(t, J=7.1Hz, 2H), 1.55–1.61(m, 2H), 1.39–1.20(m, 2H), 0.90(t, J=7.4Hz, 3H).13CNMR(126MHz, DMSO)δC: 166.51, 163.86, 144.63, 136.54, 134.05, 128.62, 127.17, 123.50, 38.13, 30.24, 19.94, 13.94.
Embodiment 2
[0024] Embodiment 2: the synthesis of compound 2
[0025]Compound 1 (0.4964g, 2mmol) obtained in the previous step was dissolved in 15ml of methanol, 10% w / w Pd / C (0.0496g, 5mol%) was added, the system was vacuumed, hydrogen was introduced and stirred, and refluxed at 65°C for 12h. After the reaction was terminated, the solid catalyst was removed by suction filtration under reduced pressure, the obtained filtrate was rotary evaporated under reduced pressure to remove the solvent, and compound 2 was obtained by separation by chromatography column. Yield: 0.6240 g. Yield: 71.5%. Compound 2 was characterized as follows: 1HNMR (500MHz, CDCl3) δ7.42 (dd, J = 8.3, 7.1Hz, 1H), 7.16 (d, J = 7.6Hz, 1H), 6.86 (d, J = 8.8Hz, 1H) ,5.13(s,1H),3.65(t,J=7.3Hz,2H),1.63–1.70(m,2H),1.35–1.42(m,2H).0.95(t,J=7.4Hz,3H). 13CNMR (126MHz, DMSO) δC: 170.38, 168.77, 145.15, 135.02, 132.86, 120.96, 112.58, 111.38, 37.37, 30.76, 20.09, 13.69.
Embodiment 3
[0026] Embodiment 3: the synthesis of compound 3
[0027] Compound 2 obtained in the previous step was dissolved in 10 ml of 50% sulfuric acid, stirred under an ice-water bath at 0°C, and aqueous sodium nitrite solution (0.0692 mg, 1 mmol, 2 mL of water) was slowly added dropwise. After continuing to stir at 0°C for 30min, the mixture was heated to 90°C and the reaction was continued for 1h. After the reaction, pour the reaction solution into 50ml of water, extract 3 times with ethyl acetate (20mL×3), wash with saturated brine, dry over anhydrous sodium sulfate, remove the solvent by rotary evaporation, and separate by column chromatography after vacuum drying Compound 3. Yield: 0.1862 g. Yield: 85.4%. Compound 3 was characterized as follows: 1HNMR (500MHz, CDCl3) δ7.70(s, 1H), 7.56(t, J=7.6Hz, 1H), 7.36(d, J=7.2Hz, 1H), 7.15(d, J= 8.4Hz, 1H), 3.65(t, J=7.3Hz, 2H), 1.68–1.62(m, 2H), 1.40–1.32(m, 2H), 0.95(t, J=7.4Hz, 3H).13CNMR( 126MHz, DMSO) δC: 170.51, 167.91, 154.60, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com