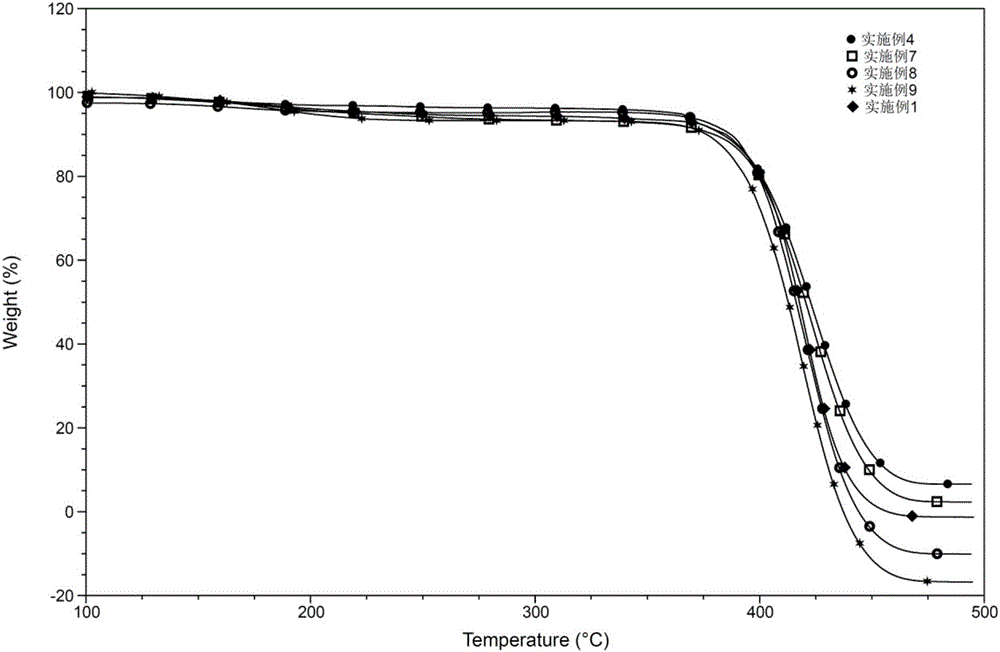
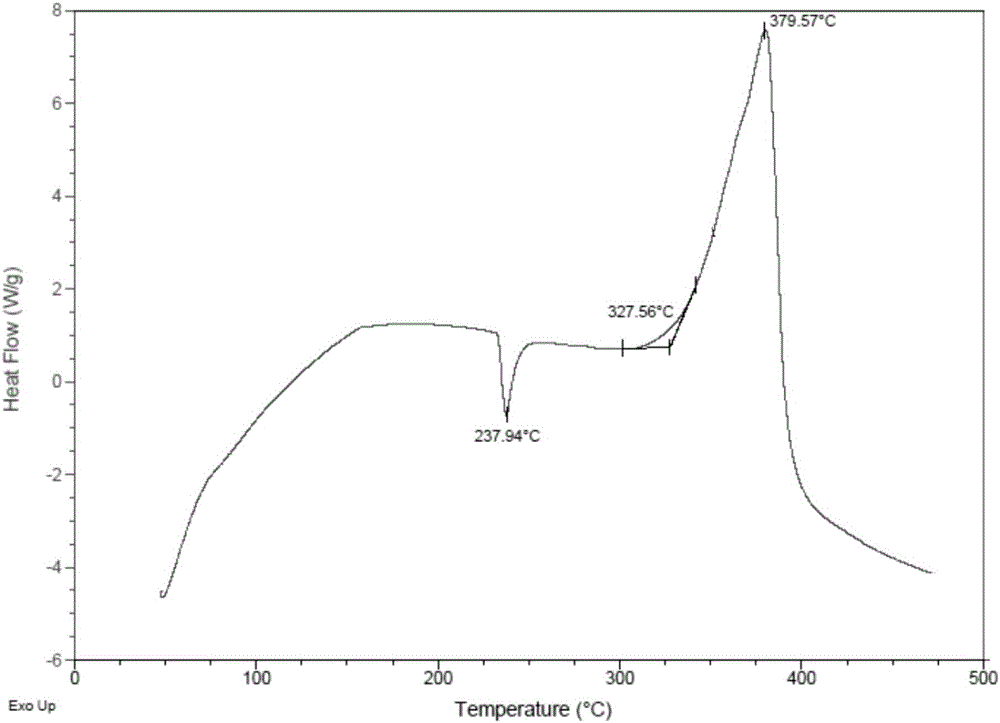
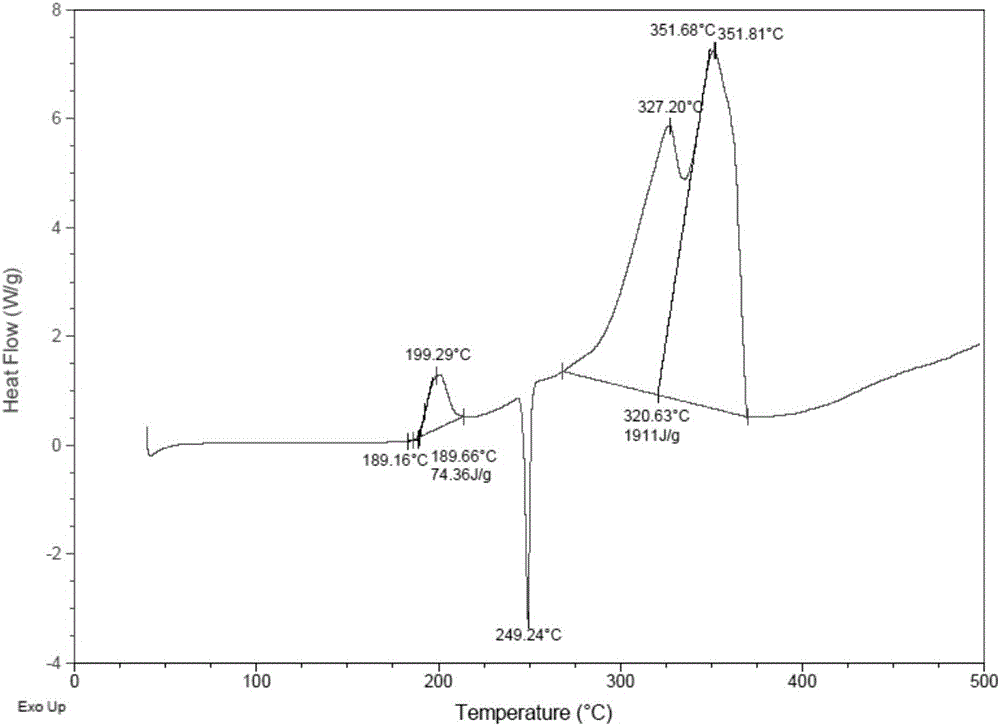
Vinylferrocene oligomer catalyst for catalyzing decomposition of ammonium perchlorate
A technology of vinyl ferrocene and ammonium perchlorate, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical/physical processes, etc., can solve volatile, ferrocene Low catalyst iron content, reduced impact sensitivity of ferrocene catalyst/ammonium perchlorate binary mixture, etc., to achieve adjustable molecular weight, significant catalytic decomposition effect, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Under anhydrous and oxygen-free conditions, vinyl ferrocene monomer (5.30 g, 25 mmol), anhydrous THF (20 mL), TMEDA (0.45 mL, 3 mmol) were added to a 100 mL Schlenk bottle. The reaction solution was frozen to -20 to -10°C, and the initiator n-butyllithium solution (0.33mL, 2.5M, 0.83mmol) was added, and the reaction solution turned blood red. After reacting for 8h, anhydrous methanol was added to the reaction system ( 1 mL), the reaction was terminated. Add anhydrous methanol (60mL) to precipitate a precipitate, filter with suction, wash the filter residue with methanol, dissolve the filter residue with dichloromethane, filter to obtain a red polymer solution, evaporate the solvent under reduced pressure, and then use an oil pump to remove the residual solvent. Obtained 4.92 g of yellow amorphous powder with a yield of 93%. Iron content is 26.2%; GPC: Mn=4851g / mol; FT-IR (KBr, mainpeaks): ν (cm -1 ) 3089, 2926, 2853, 1629, 1458, 1411, 1397, 1338, 1106, 1068, 1041, 102...
Embodiment 2
[0022] Only change the amount of n-butyllithium solution in Example 1 to (5mL, 2.5M, 12.5mmol), and other operations are consistent with Example 1. 5.25 g of yellow amorphous powder was obtained with a yield of 100%. Iron content 24.4%; GPC: Mn=442 g / mol. FT-IR (KBr, mainpeaks): ν (cm -1 ) 3089, 2925, 2852, 1632, 1458, 1411, 1338, 1106, 1054, 1037, 1023, 1000, 915, 815, 483; 1 HNMR (300MHz, CDCl 3 )δ4.65~3.90(m, Cp), 3.25~0.75(m, CH-CH 2 ); 13 CNMR (101MHz, CDCl 3 )δ67.66 (Cp), 43.27 (CH-CH 2 ), 31.86 (CH-CH 2 ), 22.77 (CH 3 -CH 2 ), 14.34 (CH 3 -CH 2 ).
Embodiment 3
[0024] Only the amount of n-butyllithium solution in Example 1 was changed to (2.5mL, 2.5M, 6.25mmol), and other operations were consistent with Example 1. 5.24 g of yellow amorphous powder was obtained with a yield of 100%. Iron content 25.7%; GPC: Mn=724 g / mol. FT-IR (KBr, mainpeaks): ν (cm -1 )3090, 2924, 2853, 1630, 1458, 1410, 1338, 1106, 1041, 1023, 999, 814, 482; 1 HNMR (300MHz, CDCl 3 )δ4.80~3.85(m, Cp), 3.25~0.80(m, CH-CH 2 ); 13 CNMR (101MHz, CDCl 3 )δ67.66 (Cp), 43.27 (CH-CH 2 ), 31.86 (CH-CH 2 ), 22.77 (CH 3-CH 2 ), 14.34 (CH 3 -CH 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com