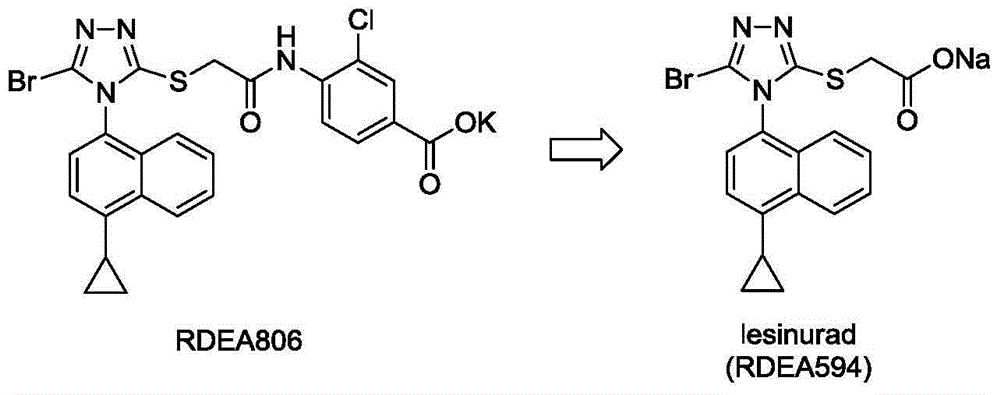
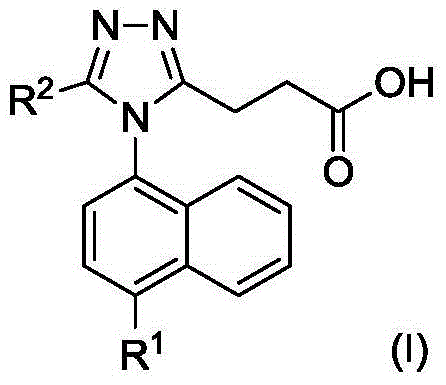
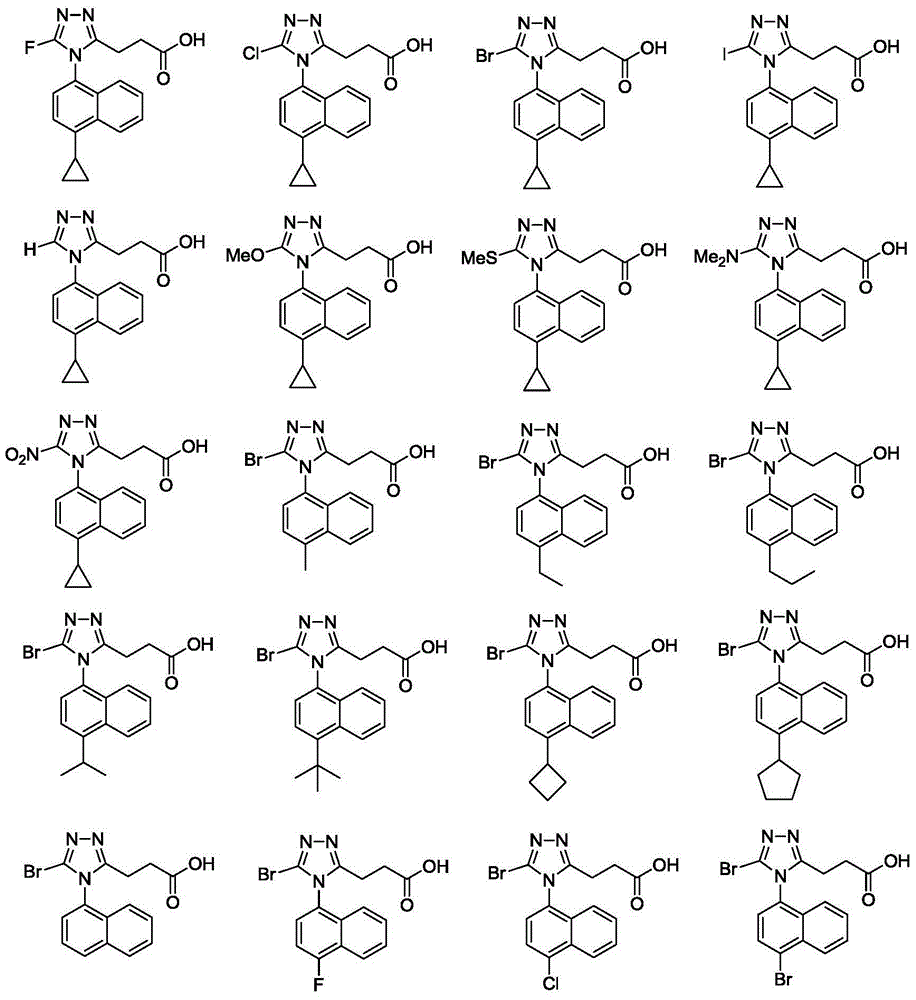
Triazole propionate URAT1 inhibitor, preparation method thereof and purpose of triazole propionate URAT1 inhibitor for treating hyperuricemia and gout
A halogenated agent, XI-A technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., can solve serious side effects, unsuitable for long-term treatment, fulminant hepatitis and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Synthesis of Compound I-1
[0031]
[0032] Step 1. Synthesis of compound VI-1
[0033] 4-pentenoyl hydrazide II is synthesized according to literature methods (Gilchrist, T.L.; etal. Synthesis, 1983, 153-154). 4-pentenoyl hydrazide II (11.41 g, 100 mmol) and N,N-dimethylformamide dimethyl acetal III (11.92 g, 100 mmol) were dissolved in 230 mL of acetonitrile, and heated at 50°C with stirring until TLC The reaction was found to be complete (usually about 0.5-1 hour).
[0034] After the reaction was completed, the reaction mixture was slightly cooled and concentrated on a rotary evaporator to 1 / 3 of its original volume, at which time an IV solution was obtained. 4-cyclopropylnaphthylamine V-1 (18.32 g, 100 mmol) and 230 mL of glacial acetic acid were added thereto, and the reaction mixture was stirred at 120° C. overnight under the protection of nitrogen. TLC inspection found that the reaction was complete.
[0035] After the reaction mixture was cooled, it was pour...
Embodiment 2
[0054] Example 2 Synthesis of Compound I-2
[0055]
[0056] Step 1. Synthesis of compound XI-A-2
[0057] Compound X-1 (0.96 g, 3 mmol) was dissolved in 10 mL of acetonitrile, stirred at room temperature, and N-chlorosuccinimide (NCS, 0.48 g, 3.6 mmol) was added. After the addition is complete, the reaction mixture is stirred at room temperature until TLC checks that the reaction is complete (usually 24 hours).
[0058] The reaction mixture was poured into 100mL ice water, stirred, and 50mL×3CH 2 Cl 2 extraction. Combine and extract the organic phases, then use Na 2 S 2 O 3 Solution, 100mL×5 saturated Na 2 CO 3 Wash solution and 5% salt water, anhydrous Na 2 SO 4 dry. The dried organic phase was evaporated on a rotary evaporator to remove the solvent, and the obtained residue was purified by column chromatography to obtain product XI-A-2, a white solid, 0.82 g, a yield of 77%. MS, m / z=356([M+H] + ).
[0059] Step 2. Synthesis of Compound I-2
[0060] Compound XI-A-2 (0.71g, 2mmol)...
Embodiment 3
[0062] Example 3 Synthesis of Compound I-3
[0063]
[0064] Step 1. Synthesis of compound XI-A-3
[0065] Compound X-1 (4.82 g, 15 mmol) was dissolved in 50 mL of acetonitrile, stirred at room temperature, and N-iodosuccinimide (NIS, 4.05 g, 18 mmol) was added. After the addition is complete, the reaction mixture is stirred at room temperature until TLC checks that the reaction is complete (usually 24 hours).
[0066] The reaction mixture was poured into 300mL ice water, stirred, 100mL×3CH 2 Cl 2 extraction. Combine and extract the organic phases, then use Na 2 S 2 O 3 Solution, 100mL×5 saturated Na 2 CO 3 Wash solution and 5% salt water, anhydrous Na 2 SO 4 dry. The dried organic phase was evaporated on a rotary evaporator to remove the solvent, and the obtained residue was purified by column chromatography to obtain product XI-A-3, a white solid, 5.43 g, yield 81%. MS, m / z=448([M+H] + ).
[0067] Step 2. Synthesis of Compound I-3
[0068] Compound XI-A-3 (0.89g, 2mmol) was disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com