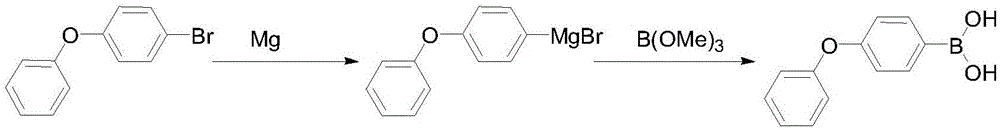
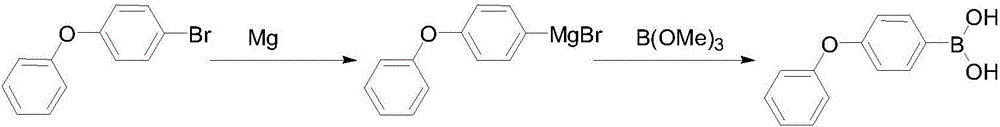
Preparation method of 4-phenoxyphenylboronic acid
A technology of phenoxybenzene boronic acid and trimethyl borate is applied in the field of preparation of 4-phenoxy benzene boronic acid, and achieves the effects of simple post-processing operation, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Grignard reagent: First, add 600mL THF and 200g 4-bromodiphenyl ether into a 1000mL three-necked flask and stir to make a solution for later use. Then add 25g of magnesium, 20mL of THF, and a small grain of iodine into a 2000mL three-necked flask, drop 20mL of the THF solution of 4-bromodiphenyl ether that has been prepared, and after heating and triggering, add the remaining 4-bromodiphenyl ether dropwise at 10-20℃ The THF solution of phenyl ether, after the dropwise addition, was kept and stirred at 30-45°C for 1 hour, and the reaction bottle was cooled to below -30°C with liquid nitrogen for later use.
[0019] (2) Boration: Slowly add 110g of trimethyl borate in 600mL THF solution dropwise to the above-mentioned format solution, the dropping process is maintained at -40~-30°C, after the dropping is completed, stir and naturally rise to room temperature, then add 400mL of 10% hydrochloric acid dropwise, Reflux for 1 hour, after THF was distilled off, 500 mL of co...
Embodiment 2
[0022] (1) Grignard reagent: First, add 600mL THF and 200g 4-bromodiphenyl ether into a 1000mL three-necked flask and stir to make a solution for later use. Then add 20.5g of magnesium, 20mL of THF, and a small grain of iodine into a 2000mL three-necked flask, drop 20mL of the prepared THF solution of 4-bromodiphenyl ether into it, and after heating and triggering, add the remaining 4-bromodiphenyl ether dropwise at 10-20°C After the THF solution of diphenyl ether is added dropwise, keep stirring at 30-45° C. for 1 hour, and cool the reaction bottle to below -30° C. with liquid nitrogen for later use.
[0023] (2) Boration: Slowly add 91.8g of trimethyl borate in 600mL THF solution dropwise to the above-mentioned format solution, keep the dropping process below -30°C, stir and naturally rise to room temperature after dropping, add 400mL of 10% hydrochloric acid dropwise, and reflux After 1 hour, THF was distilled off, 500 mL of cold water was added, stirred for 30 minutes, coo...
Embodiment 3
[0026] (1) Grignard reagent: First, add 600mL THF and 200g 4-bromodiphenyl ether into a 1000mL three-necked flask and stir to make a solution for later use. Then add 20.0g of magnesium, 20mL of THF, and a small grain of iodine into a 2000mL three-necked flask, drop 20mL of the prepared THF solution of 4-bromodiphenyl ether into it, and after heating and triggering, add the remaining 4-bromodiphenyl ether dropwise at 45-50°C After the THF solution of diphenyl ether is added dropwise, keep stirring at 45-50° C. for 1 hour, and cool the reaction bottle to below -30° C. with liquid nitrogen for later use.
[0027] (2) Boration: Slowly add 91.8g of trimethyl borate in 600mL THF solution dropwise to the above-mentioned format solution, keep the temperature below -50~-40°C during the dropwise addition process, stir and naturally rise to room temperature after dropping, add 400mL10% solution dropwise Hydrochloric acid was refluxed for 1 hour. After THF was distilled off, 500 mL of col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com