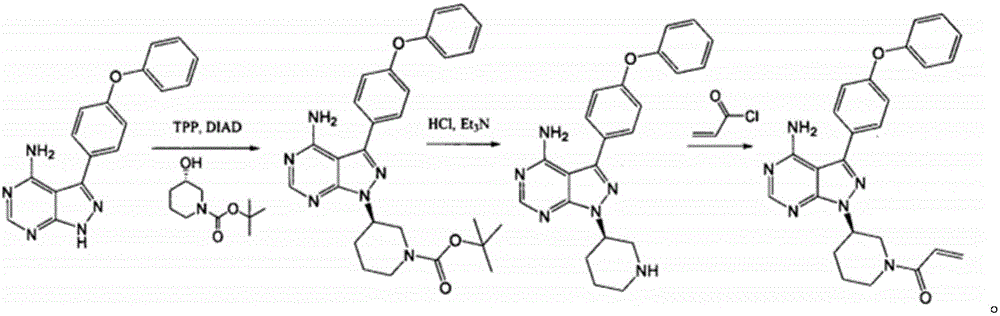
Carbonyl reductase and application thereof in preparation of Chiral N-protecting-hydroxy nitrogen heterocycle
A carbonyl reductase and reductase technology, applied in the biological field, can solve the problems of limiting the application range of carbonyl reductase, narrow substrate spectrum, high price, etc., and achieve the effects of good industrial application prospect, wide substrate spectrum and high activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Screening of highly active N-Boc-carbonylpiperidine reductase mutants
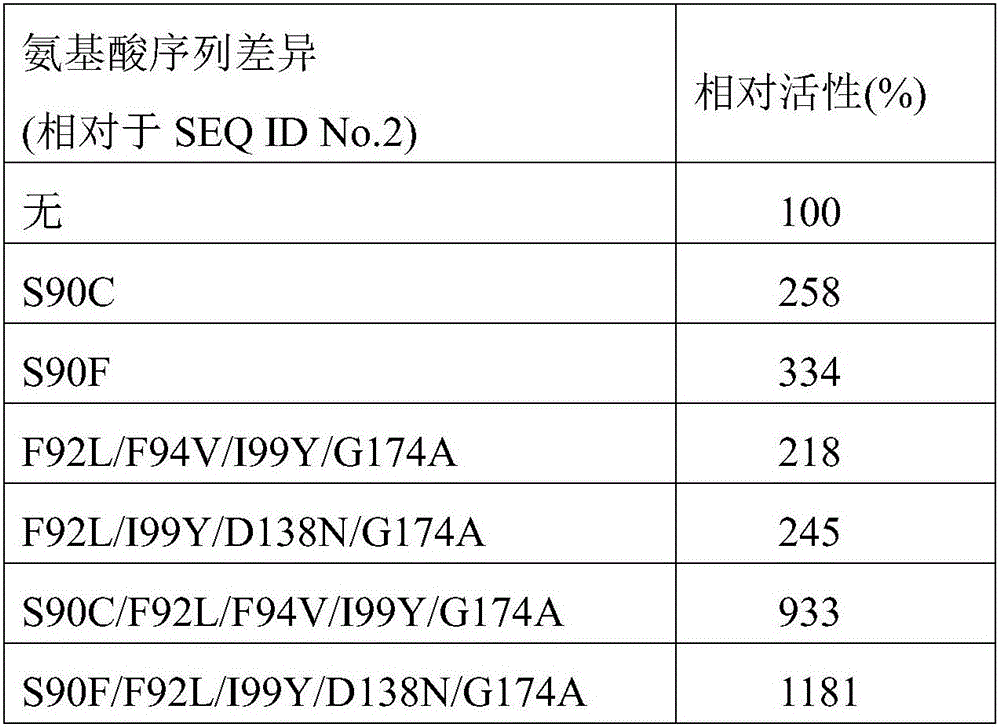
[0032] The carbonyl reductase CgKR1 of the amino acid sequence shown in the sequence table SEQIDNo.2 was mutated and transformed by using error-prone PCR strategy to improve the reactivity of the enzyme to N-Boc-carbonylpiperidine and N-Boc-carbonylpyrrole, and found that 90 Serine residues are key sites that affect the activity of CgKR1.
[0033] On this basis, different mutants of serine at position 90 were combined with the published mutations of F92L, F94V, I99Y, D138N, and G174A, and the obtained combined mutants were screened for activity, and two mutants with significantly improved activity were obtained CgKR1 S90C / F92L / F94V / I99Y / G174A and CgKR1 S90F / F92L / I99Y / D138N / G174A .
[0034] The mutants with significantly improved activity obtained through screening are shown in Table 1.
[0035] Table 1 N-Boc-carbonyl piperidine reducing activity improved carbonyl reductase mutants
[...
Embodiment 2
[0038] Example 2 Recombinase CgKR1 S90C / F92L / F94V / I99Y / G174A Fermentation production of
[0039] Recombinase CgKR1 S90C / F92L / F94V / I99Y / G174A In the amino acid sequence shown in SEQIDNo.2, the 90th serine is replaced by cysteine, the 92nd phenylalanine is replaced by leucine, the 94th phenylalanine is replaced by valine, and the 99th Carbonyl reductase consisting of a new amino acid sequence formed by replacing isoleucine with tyrosine and glycine at position 174 with alanine.
[0040] Recombinase CgKR1 S90C / F92L / F94V / I99Y / G174A It is obtained by recombinant gene engineering Escherichia coli through fermentation and expression.
[0041] The enzyme was fermented in a 5L fermenter, and the fermentation medium was formulated with 2×LB, that is, 20g / L peptone, 10g / L yeast powder and 10g / L NaCl. The feed nitrogen source is a mixture of 60 g / L peptone and 60 g / L yeast powder, and the carbon source is 50% (w / w) glycerol. The pH of fermented liquid is added acid (massfraction 20% ...
Embodiment 3
[0042] Example 3 Recombinase CgKR1 S90F / F92L / I99Y / D138N / G174A Fermentation production of
[0043] Recombinase CgKR1 S90F / F92L / I99Y / D138N / G174A In the amino acid sequence shown in SEQIDNo.2, the 90th serine is replaced by phenylalanine, the 92nd phenylalanine is replaced by leucine, the 99th isoleucine is replaced by tyrosine, and the 138th A carbonyl reductase consisting of a new amino acid sequence formed by replacing aspartic acid with asparagine and replacing glycine at position 174 with alanine.
[0044] Recombinase CgKR1 S90F / F92L / I99Y / D138N / G174A It is obtained by recombinant gene engineering Escherichia coli through fermentation and expression.
[0045] The enzyme was fermented in a 5L fermenter, and the fermentation medium was formulated with 2×LB, that is, 20g / L peptone, 10g / L yeast powder and 10g / L NaCl. The feeding nitrogen source was 60g / L peptone and 60g / L yeast powder, and the carbon source was 50% (w / w) glycerin. The pH of fermented liquid is added acid (ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com