Xylanase thermohaline modified mutant and application thereof
A technology of xylanase and mutant, applied in the field of genetic engineering and protein modification, can solve the problems of inactivity, inactive thermostability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Construction of mutant library
[0033] ① The genomes of Arthrobacter sp. and Lechevalieria sp. were extracted according to the instructions of the GENESTAR Bacterial Genome Extraction Kit.
[0034] ②According to the Arthrobacter sp. xylanase nucleotide sequence JQ863105 (SEQ ID No.3) recorded in GenBank, primers 5'GTGCAGCCGGAGGAAAAACG3' and 5'GATGAAGGCAGGATCCGGGGT3' were designed, and the Arthrobacter sp. genome was used as a template for PCR Amplify to obtain the xynAGN16L xylanase gene; in addition, according to the Lechevalieria sp. xylanase nucleotide sequence JF745868 (SEQ ID No.4) recorded in GenBank, design primers 5'GTCTCGGCCCCGCCGGACGT3' and 5' GGCTCGCTTCGCCAGCGTGG3', the xylanase gene xynAHJ3 was obtained by performing PCR amplification using the Lechevalieria sp. genome as a template.
[0035] ③PCR reaction parameters are: denaturation at 94°C for 5min; then denaturation at 94°C for 30sec, annealing at 55°C for 30sec, extension at 72°C for 1min30...
Embodiment 2
[0041] Example 2: Screening of Mutants
[0042] 1) Take 2 μL of bacterial liquid from the 96-well cell culture plate where the mutant library is stored, and inoculate it into 200 μL / well liquid LB culture medium (containing 100 μgmL -1 Amp) in a 96-deep-well plate at 37°C with shaking at 200rpm until OD 600 >1.0 (about 20h), add 2mMIPTG and 100μgmL -1 Amp was induced overnight at 20° C. with 160 rpm in 200 μL liquid LB culture solution.
[0043] 2) Add 40 μL / well of PopCulture after induction TM Cell lysate, shake and lyse cells at 25°C for 30 minutes.
[0044] 3) Take 50 μL of McIlvaine buffer (pH=7.0) containing 1.0% (w / v) beech xylan and 50 μL of cell lysate, and react in a 96 deep-well plate in a 70° C. incubator for 2 hours. After the reaction is over, add 150 μL of DNS reagent to terminate the reaction, incubate in a 140°C incubator for more than 20 minutes and cool to room temperature, and use a microplate reader to read the OD 540nm The value of E.coliBL21-Gold (D...
Embodiment 3
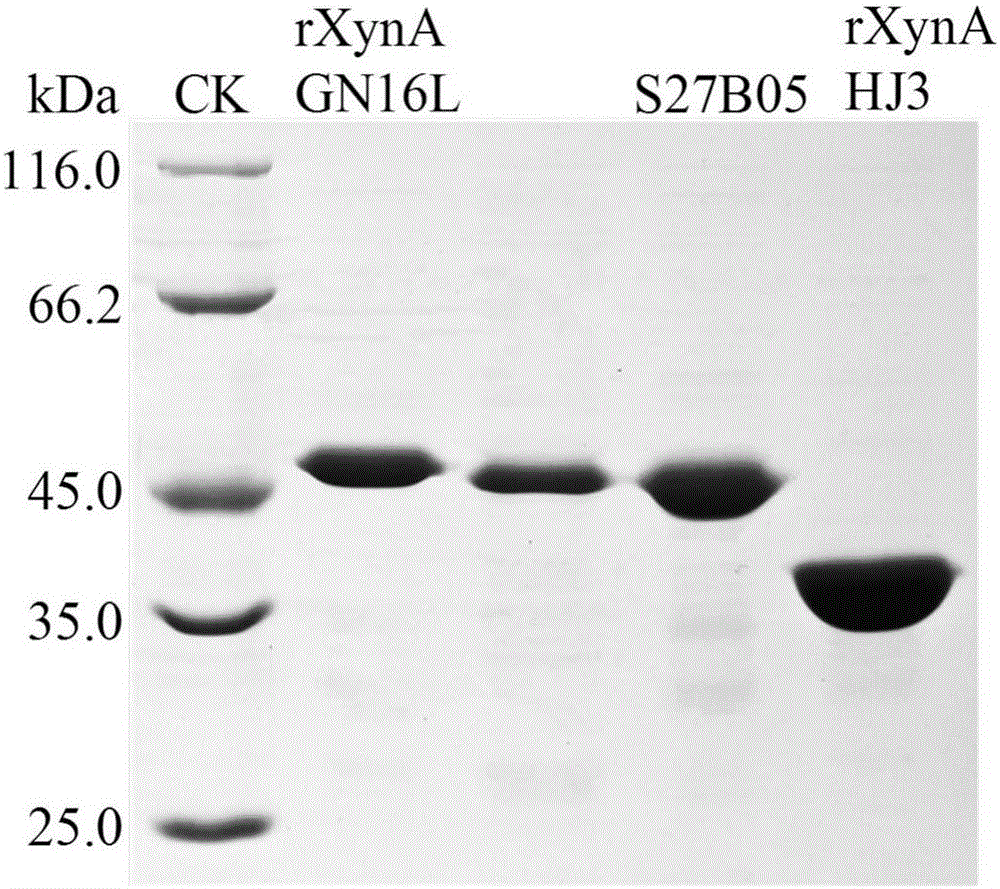
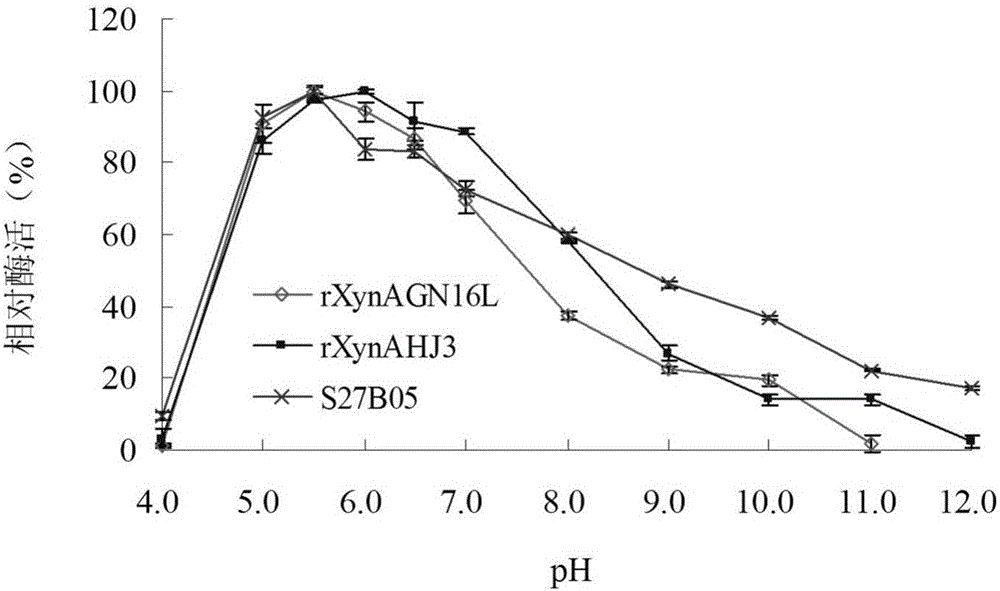
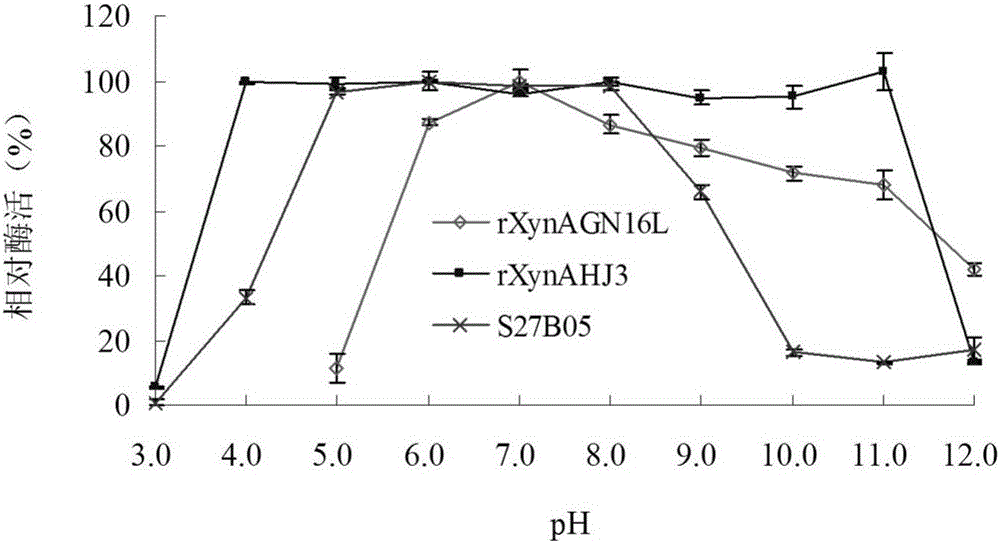
[0048] Embodiment 3: Enzyme preparation of mutant S27B05 and wild enzyme rXynAGN16L and rXynAHJ3
[0049] The recombinant strains containing mutant S27B05, wild enzymes rXynAGN16L and rXynAHJ3 were inoculated in LB (containing 100 μg mL -1 Amp) medium, shake rapidly at 37°C for 16h.
[0050] Then this activated bacterial solution was inoculated into fresh LB (containing 100 μg mL -1 Amp) culture medium, rapid shaking culture for about 2–3h (OD 600 After reaching 0.6-1.0), add IPTG at a final concentration of 0.1 mM for induction, and continue shaking culture at 20° C. for about 20 h. Centrifuge at 12000rpm for 5min to collect the bacteria. After suspending the bacteria with an appropriate amount of pH=7.0 Tris-HCl buffer solution, the bacteria were disrupted ultrasonically in a low-temperature water bath. The crude enzyme solution concentrated in the cells above was centrifuged at 13,000rpm for 10min, the supernatant was aspirated, and the target protein was affinity-purif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com