A kind of preparation method of JAK inhibitor momelotinib
An inhibitor and organic solvent technology, applied in the field of small molecule chemical drug preparation, can solve the problems of restricting the industrialized production of compounds, numerous reaction routes, complicated by-products, etc., and achieve the effects of low cost, simple process steps and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
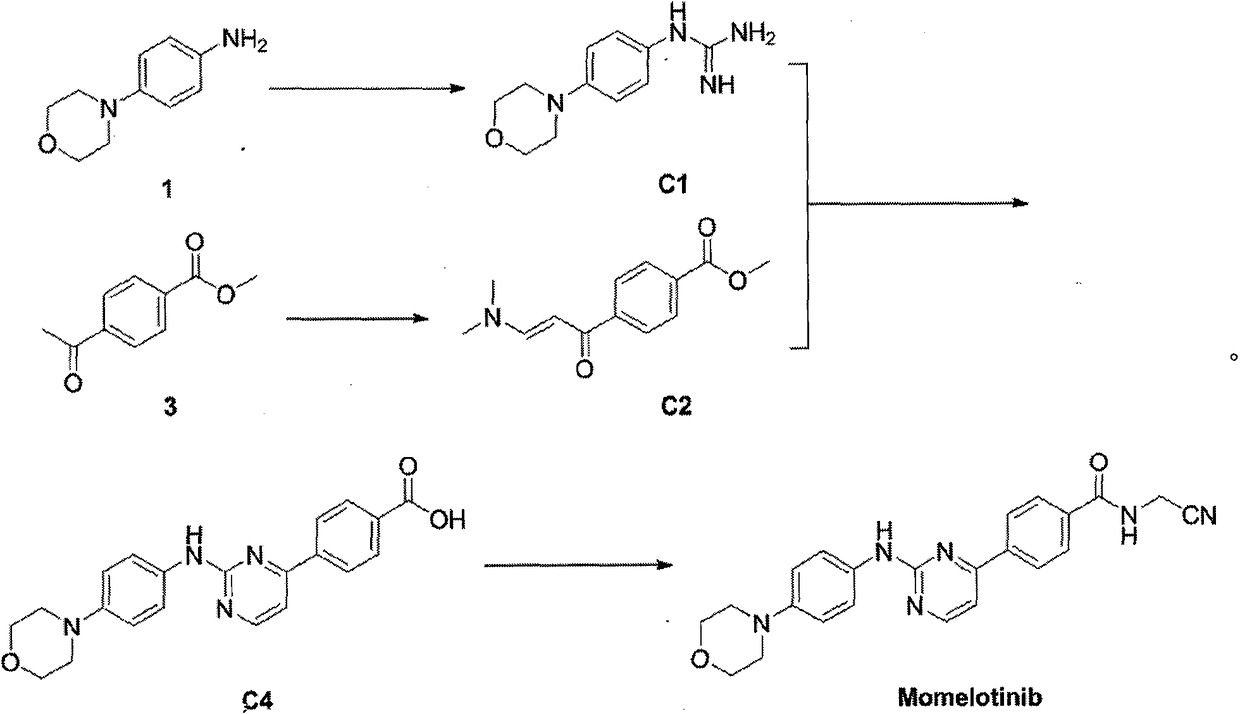
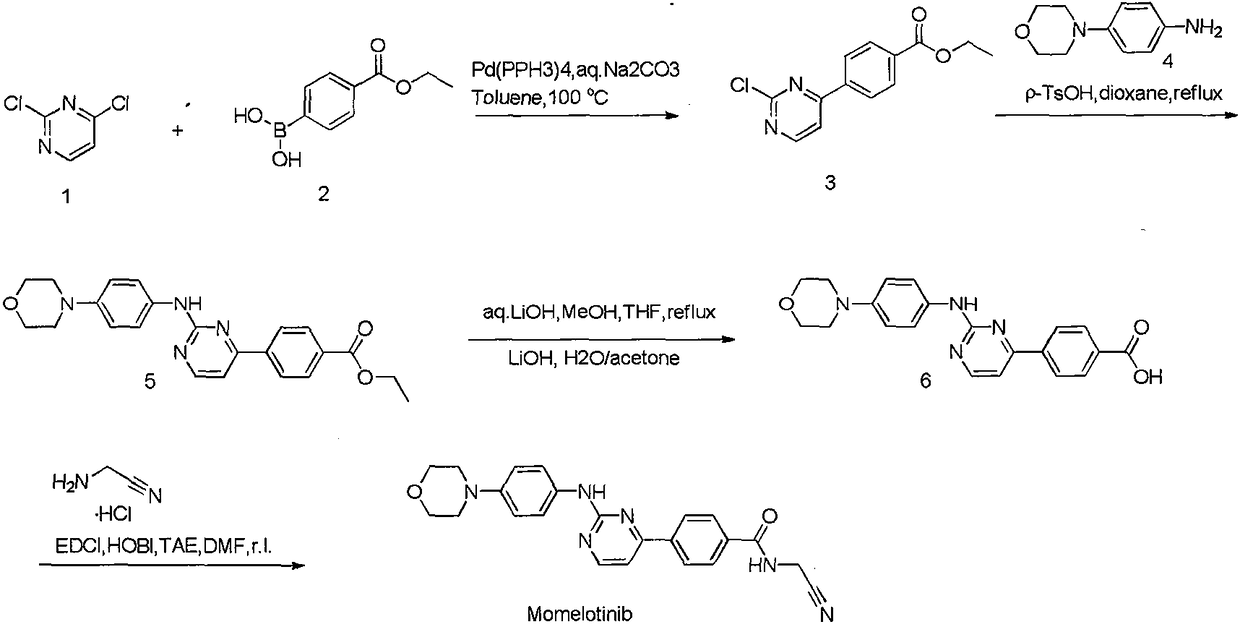
[0032] Preparation of intermediate C1
[0033]
[0034] Dissolve 1.78g (10mmol) of 4-morpholine-aniline and 1.7ml (20mmol) of 50% mononitrile amine aqueous solution in 50ml of absolute ethanol, add 1.5ml of concentrated hydrochloric acid dropwise, and heat to reflux at 80-90°C for 2h , and then add concentrated hydrochloric acid 1.5ml, continue to react for 8h. Concentrate to remove ethanol, add sodium carbonate aqueous solution to precipitate solid, filter, and wash the filter cake with acetone to obtain 2.3 g of white solid with a yield of 82%.
[0035] The data of the HNMR of target product intermediate C2 are as follows:
[0036] 1 H NMR (300MHz, DMSO-d6): δ7.16(brs, 2H), 6.88(m, 4H), 6.59(brs, 1H), 4.25(brs, 1H), 3.72(m, 4H), 3.04(m ,4H); 13 C NMR (75MHz, DMSO-d6): δ160.0, 154.9, 147.7, 124.7, 116.1, 66.1, 48.9.
Embodiment 2
[0038] Preparation of Intermediate C2
[0039]
[0040] 2g (10mmol) of methyl p-acetylbenzoate was added to 9ml (60mmol) of DMF-DMA solution, heated to reflux at 85°C for 15h, and filtered to obtain 2.3g of a yellow solid with a yield of 86%.
[0041] The data of the HNMR of target product intermediate C2 are as follows:
[0042] 1H NMR (300MHz, DMSO-d6): δ7.99(m, 4H), 7.76(d, J=12Hz, 1H), 5.84(d, J=12Hz, 1H), 3.88(s, 3H), 3.17( s, 3H), 2.94(s, 3H); 13 C NMR (75MHz, DMSO-d6): δ184.6, 165.8, 154.7, 144.2, 131.1, 128.9, 127.3, 91.0, 52.2, 44.5, 37.2.
Embodiment 3
[0044] Preparation of intermediate C4
[0045]
[0046] Add 1.68g (0.6mmol) of intermediate C1 (0.6mmol) and 1.16g (0.5mmol) of C2 to 35ml of n-butanol, then add 0.8g (10mmol) of sodium hydroxide, heat to 98°C for 24h under reflux, and get yellow after washing with ether solid. Dissolve 0.58 g of the obtained solid in 10 ml of acetone, add 0.12 g of lithium hydroxide in 1.6 ml of aqueous solution, react overnight at room temperature, concentrate to remove the organic solvent, adjust the pH to acidic with dilute hydrochloric acid, precipitate the solid, and filter to obtain intermediate C4 , two-step yield of 85%.
[0047] The data of the HNMR of target product intermediate C4 is as follows:
[0048] 1 H NMR (300MHz, DMSO-d6): δ9.45(s, 1H), 8.53(d, J=5.1Hz, 1H), 8.23(d, J=8.1Hz, 2H), 8.09(d, J=8.1 Hz, 2H), 7.67(d, J=8.7Hz, 2H), 7.37(d, J=5.1Hz, 1H), 6.93(d, J=8.7Hz, 2H), 3.75(m, 4H), 3.06( m,4H); 13 C NMR (75MHz, DMSO-d6): δ162.6, 160.3, 159.2, 146.2, 140.4, 132.8, 129...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com