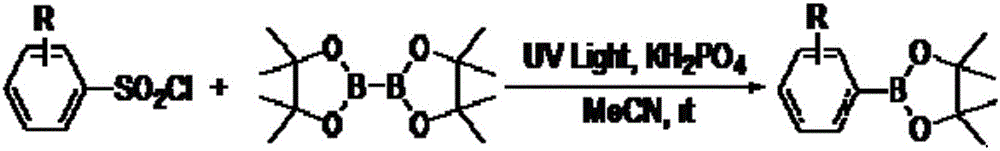
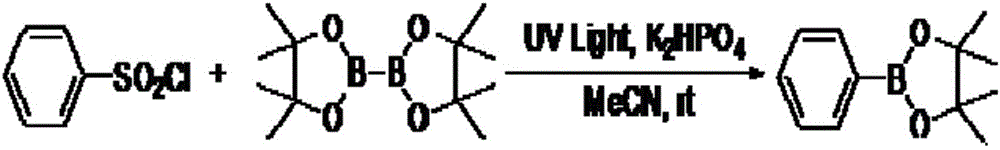
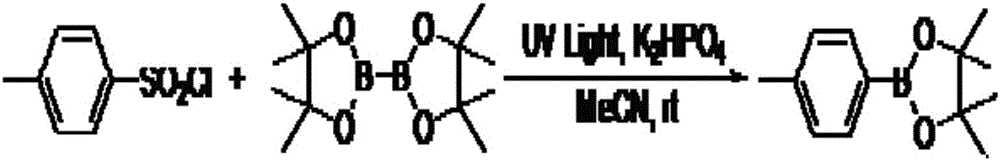Synthesis method of aromatic borate compounds
The technology of an aromatic boronate ester and a synthesis method is applied in the field of using ultraviolet light to induce the synthesis of aromatic boronate ester compounds, which can solve the problems such as no literature report, and achieve the effects of high yield, economical raw materials and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: the synthesis of phenylboronic acid ester
[0015]
[0016] Add 2mL of acetonitrile, benzenesulfonyl chloride (52.8mg, 0.3mmol), pinacol diborate (114.3mg, 0.45mmol), dipotassium hydrogen phosphate (104.4mg, 2.0eq.) into a 25mL reaction tube, and use a UV lamp The reaction tube was irradiated and the reaction was stirred magnetically at room temperature for 24 hours. After the reaction was completed, most of the solvent was evaporated under reduced pressure, and the remaining mixed solution was separated and purified by column chromatography with petroleum ether / ethyl acetate (30:1) as the eluent to obtain the desired product as a white solid , 39.8mg, yield 65%.
[0017] Its NMR data are as follows:
[0018] 1 H NMR (300MHz, CDCl 3 )δ=7.94~7.90(m,1H),7.84(d,J=6.0Hz,1H),7.64(d,J=6.0Hz,1H),7.50~7.28(m,2H),1.37(s,12H ). 13 C NMR (75MHz, CDCl 3 )δ=137.6, 134.7, 131.2, 127.7, 127.1, 83.8, 24.9.
Embodiment 2
[0019] Embodiment 2: the synthesis of 4-methylphenyl borate
[0020]
[0021] Add acetonitrile 2mL, 4-methylbenzenesulfonyl chloride (57.0mg, 0.3mmol), diboronic acid pinacol ester (114.3mg, 0.45mmol), dipotassium hydrogen phosphate (104.4mg, 2.0eq.) into a 25mL reaction tube , the reaction tube was irradiated with ultraviolet light, and the reaction was stirred by magnetic force at room temperature for 24 hours. After the reaction was completed, most of the solvent was evaporated under reduced pressure, and the remaining mixed solution was separated and purified by column chromatography with petroleum ether / ethyl acetate (30:1) as the eluent to obtain the desired product as a white solid , 44.5 mg, yield 68%.
[0022] Its NMR data are as follows:
[0023] 1 H NMR (300MHz, CDCl 3 )δ=7.81(d, J=9.0Hz, 2H), 7.27(d, J=9.0Hz, 2H), 2.44(s, 3H), 1.41(s, 12H). 13 C NMR (75MHz, CDCl 3 )δ=141.4, 134.9, 128.6, 83.6, 24.9, 21.8.
Embodiment 3
[0024] Embodiment 3: the synthesis of 4-methoxyphenyl borate
[0025]
[0026] Add acetonitrile 2mL, 4-methylbenzenesulfonyl chloride (61.8mg, 0.3mmol), diboronic acid pinacol ester (114.3mg, 0.45mmol), dipotassium hydrogen phosphate (104.4mg, 2.0eq.) into a 25mL reaction tube , the reaction tube was irradiated with ultraviolet light, and the reaction was stirred by magnetic force at room temperature for 24 hours. After the reaction was completed, most of the solvent was evaporated under reduced pressure, and the remaining mixed solution was separated and purified by column chromatography with petroleum ether / ethyl acetate (20:1) as the eluent to obtain the desired product as a white solid , 49.2 mg, yield 70%.
[0027] Its NMR data are as follows:
[0028] 1 H NMR (300MHz, CDCl 3 )δ=7.81(d, J=9.0Hz, 2H), 6.92(d, J=9.0Hz, 2H), 3.83(s, 3H), 1.36(s, 12H). 13 C NMR (75MHz, CDCl 3 )δ=162.2, 136.5, 113.3, 83.5, 55.0, 24.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com