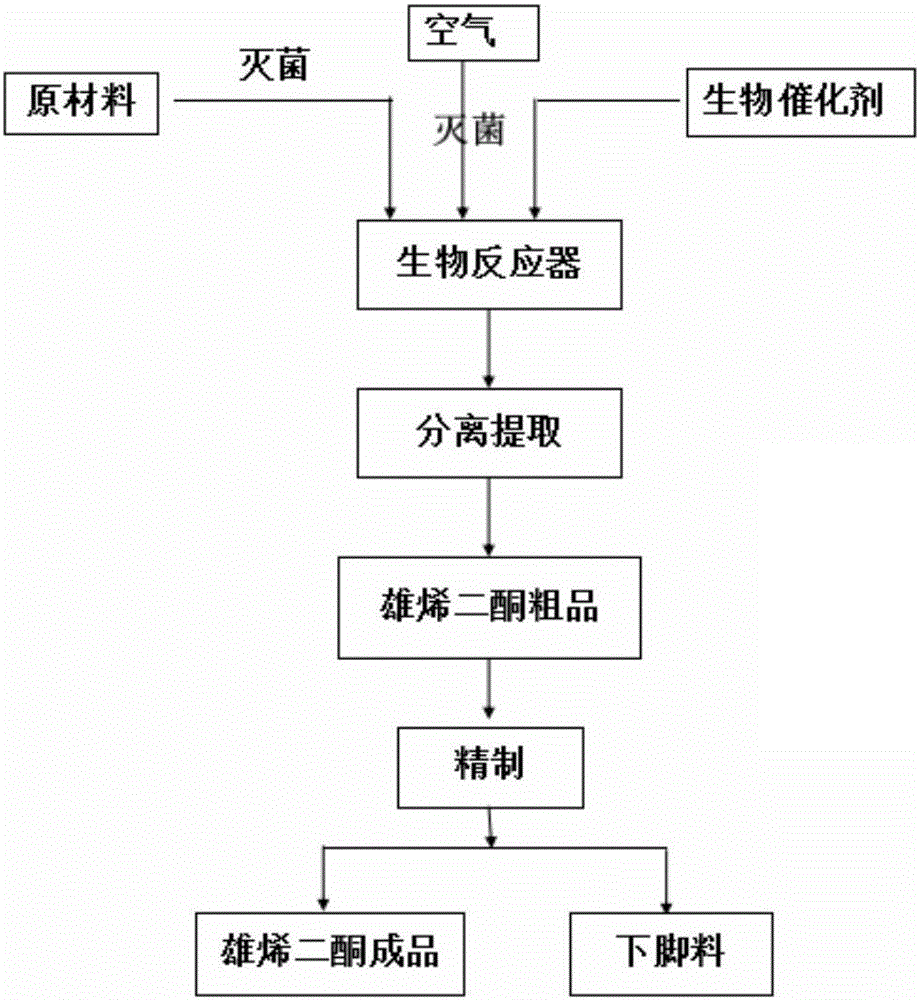
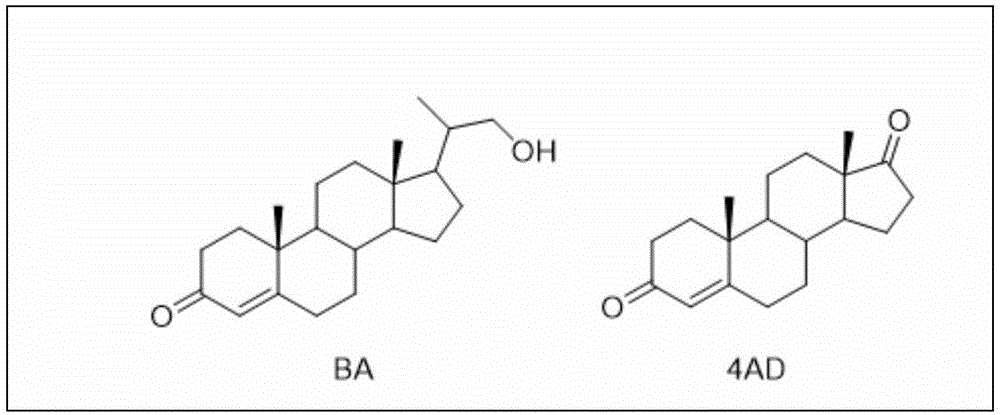
Process for recovering steroids from residues in biological fermentation based androstenedione production
A technology of steroidal compounds and bio-fermentation, which is applied in the field of production of steroidal hormone raw material drugs, can solve the problems of large soil, water pollution, lower recovery rate, and warehouse occupation, so as to increase economic benefits, high recovery rate, The effect of reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take 5.0 g of solid offal, add 15 mL of methanol, heat up to 50° C., stir to reach a dissolution equilibrium, filter, and dry the filter cake to obtain steroid compound P1. Then concentrate the supernatant, remove a part of methanol to obtain about 8mL of clear solution, slowly add water (about 8mL) dropwise thereto until the solution appears a little turbid, let it stand for 1-24h, separate layers, separate liquids to remove the lower oil, and remove the upper layer The emulsion was allowed to stand overnight, a solid precipitated out, filtered, and the filter cake was dried to obtain the steroidal compound P2. First add methanol (10 mL) to the oily substance in the lower layer to dissolve, then add water (10 mL) to precipitate a precipitate, filter, and dry the filter cake to obtain steroid compound P3. The products were combined to obtain a pale yellow steroid compound (1.5 g). Through HPLC analysis, the purity of androstenedione in the steroid compound is 75%, and ...
Embodiment 2
[0032] Take 50.0 g of solid waste, add 200 mL of ethanol, stir at room temperature to reach a dissolution equilibrium, filter, and dry the filter cake to obtain steroid compound P1. Then concentrate the supernatant, remove a part of ethanol to obtain about 100mL of clear liquid, slowly add water (about 120mL) dropwise to it until the solution appears a little turbid, let it stand for 1-24h, separate layers, separate liquid to remove the lower oil, and remove the upper layer The emulsion was allowed to stand overnight, a solid precipitated out, filtered, and the filter cake was dried to obtain the steroidal compound P2. First add ethanol (100 mL) to the oily substance in the lower layer to dissolve, then add water (100 mL) to precipitate a precipitate, filter, and dry the filter cake to obtain steroid compound P3. The products were combined to obtain a light yellow steroid compound (10.2g). Through HPLC analysis, the purity of androstenedione in the steroid compound is 88%, an...
Embodiment 3
[0034] Take 5.0 g of solid offal, add 25 mL of ethyl acetate, heat up to 30° C., stir to reach a dissolution equilibrium, filter, and dry the filter cake to obtain steroid compound P1. Then concentrate the supernatant, remove a part of ethyl acetate to obtain about 15mL of clear liquid, slowly add cyclohexane (about 20mL) dropwise to it until the solution becomes slightly turbid, let it stand for 1-24h, separate layers, and remove the lower layer by liquid separation For oily matter, the upper milky liquid was left to stand overnight, and solids were precipitated, filtered, and the filter cake was dried to obtain steroidal compound P2. Ethyl acetate (20 mL) was first added to the oily substance in the lower layer to dissolve, then cyclohexane (20 mL) was added to precipitate a precipitate, filtered, and the filter cake was dried to obtain steroid compound P3. The products were combined to obtain a pale yellow steroid compound (1.3 g). Through HPLC analysis, the purity of andr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com