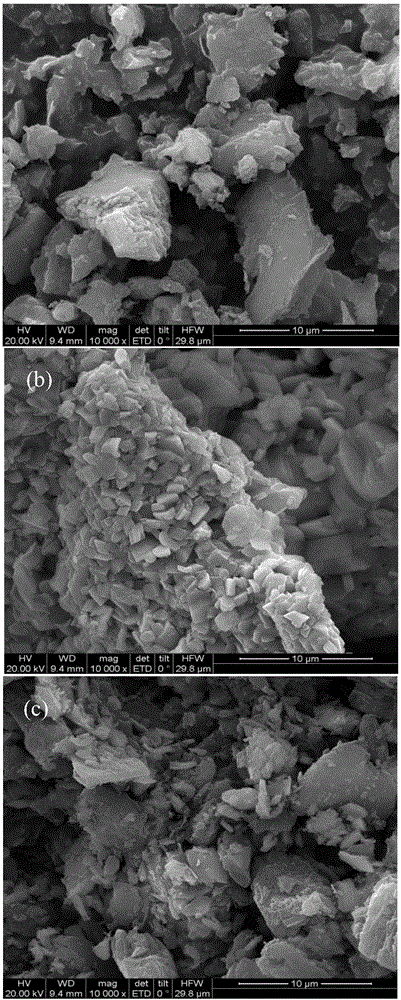
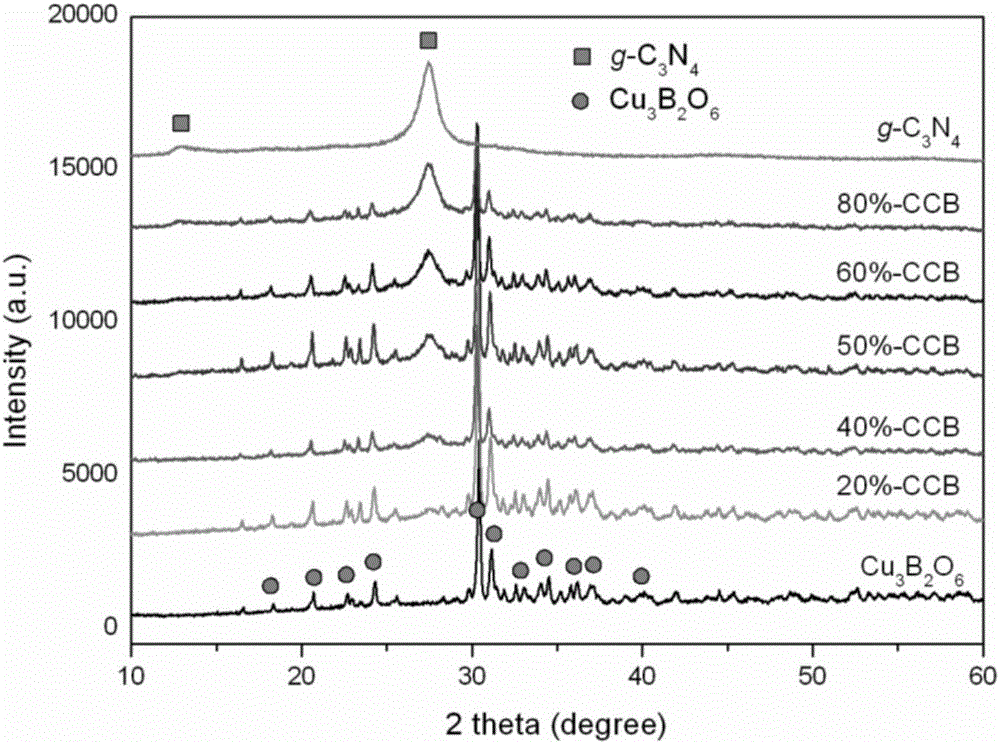
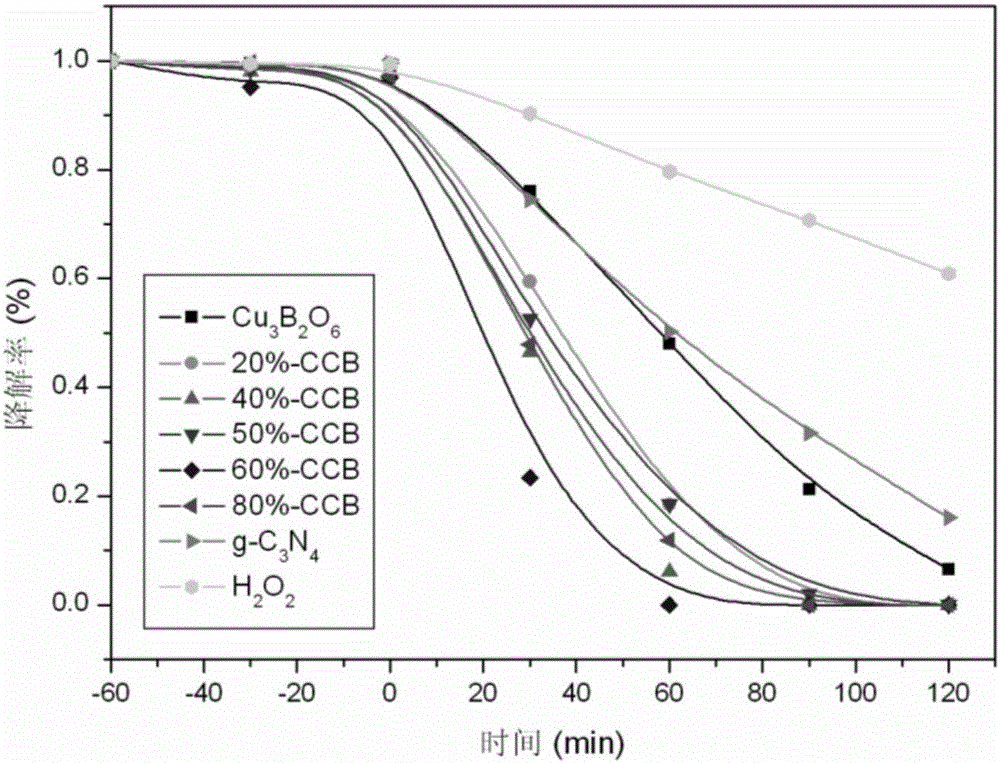
Preparation method of Cu3B2O6/g-C3N4 (cupric borate/graphitic carbon nitride) heterojunction photocatalyst and method for degrading methylene blue dye wastewater
A photocatalyst, g-c3n4 technology, applied in physical/chemical process catalysts, chemical instruments and methods, water/sludge/sewage treatment, etc., can solve the problems of low utilization rate of visible light, easy recombination, low quantum efficiency, etc. Achieve the effect of improving visible light catalytic efficiency, enhancing absorption, and improving quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Weigh 0.4991g of copper acetate and dissolve in 10mL of deionized water, then weigh 1.0507g of citric acid and dissolve in 10mL of deionized water, then slowly add the citric acid solution dropwise into the copper acetate solution, stir magnetically for 2 hours to make the copper ions and lemon Acid complexation is complete. Then, 40 mL of boric acid solution dissolved in 2.5 mmol was slowly added dropwise, and a uniform transparent blue sol was obtained after fully stirring for 2 hours. The sol was baked in an oven at 150° C. for 10 hours, and the water was evaporated to dryness to obtain a brown xerogel. Grind the xerogel into powder, move it to a muffle furnace, react in an air atmosphere at 900°C for 3 hours, take it out and grind it, and continue to react at 900°C for 2 hours. After the reaction, grind to obtain dark green Cu 3 B 2 o 6 powder. Put 5 g of melamine in a porcelain crucible with a cover, raise the temperature to 550° C. at a rate of 10° C. / min in a...
Embodiment 2
[0040] Weigh 0.04g of photocatalytic material (g-C 3 N 4 content of 40%) was dispersed in 100mL of 15mg / L methylene blue solution. Before light exposure, the catalyst-containing suspension was continuously stirred for 40 min in the dark to ensure that the adsorption-desorption equilibrium between the catalyst and the degradation pollutants was reached, and then 0.4 mL of HO was added dropwise. 2 o 2 , the sample concentration was taken as the initial concentration for degradation. During the photoreaction process, the methylene blue solution containing the catalyst was kept in a continuous stirring state, and samples were taken at regular intervals to measure the change of the methylene blue concentration with the reaction time. The experimental results showed that the concentration of methylene blue decreased by 81% after 60 minutes of light, and degraded after 90 minutes Completely, the solution becomes clear.
Embodiment 3
[0042] Weigh 0.10g of photocatalytic material (g-C 3 N 4 content of 60%) was dispersed in 100mL of 50mg / L methylene blue solution. Before light exposure, the catalyst-containing suspension was continuously stirred for 60 min in the dark to ensure that the adsorption-desorption equilibrium between the catalyst and the degradation pollutants was reached, and then 0.5 mL of H 2 o 2 , the sample concentration was taken as the initial concentration for degradation. During the photoreaction process, the methylene blue solution containing the catalyst was kept in a continuous stirring state, and samples were taken at regular intervals to measure the change of the methylene blue concentration with the reaction time. The experimental results showed that the concentration of methylene blue decreased by 62% after 60 minutes of light, and degraded after 100 minutes Completely, the solution becomes clear.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com