Fe<3+> molecular fluorescence sensor based on Rhodamine B and preparation method and application of Fe<3+> molecular fluorescence sensor
A fluorescent sensor and reaction technology, applied in the field of biochemistry, can solve the problems of poor probe sensitivity, high fluorescence intensity, and low production cost, and achieve the effects of high quantum yield, simple synthesis steps, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of fluorescent chemical sensors
[0045] 1. Synthesis of compound 1
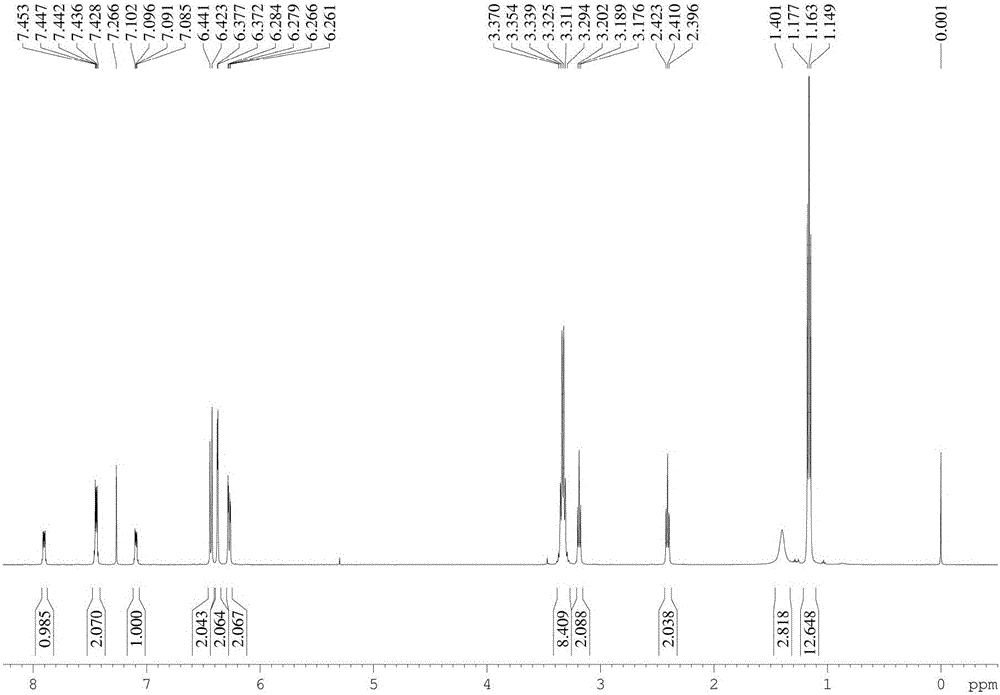
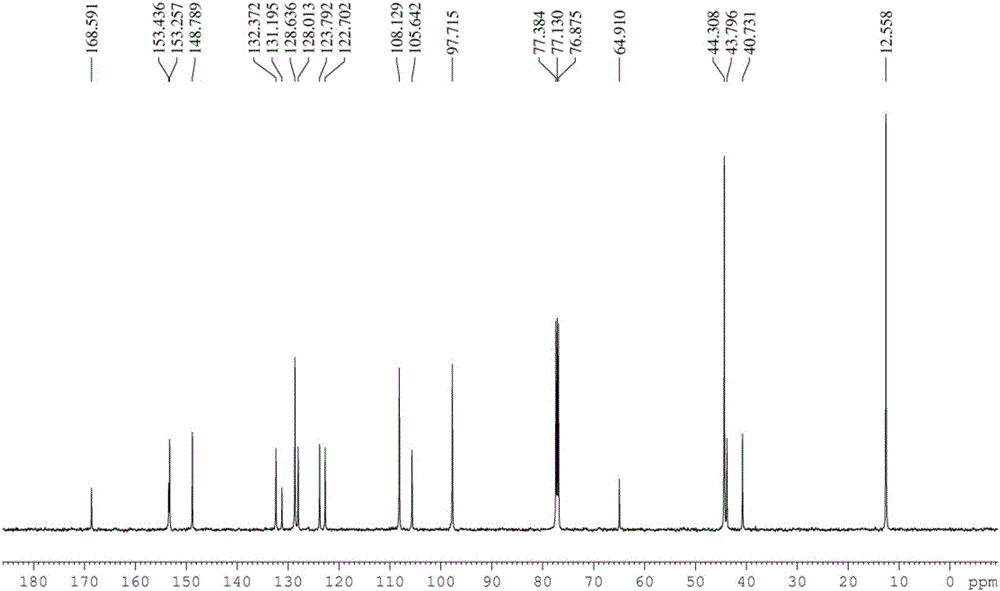
[0046] Rhodamine B (960mg, 2mmol) and ethylenediamine (0.65ml, 10mmol) were dissolved in absolute ethanol (40ml), the reaction temperature was controlled at 80°C, and the reaction time was 12h. After the reaction was completed, the solvent was removed under reduced pressure, and extracted , separated by silica gel column to obtain light yellow solid (880 mg, 92%). Compound 1 1 H NMR, 13 C NMR respectively as figure 1 , figure 2 shown.
[0047] 2. Synthesis of compound 2
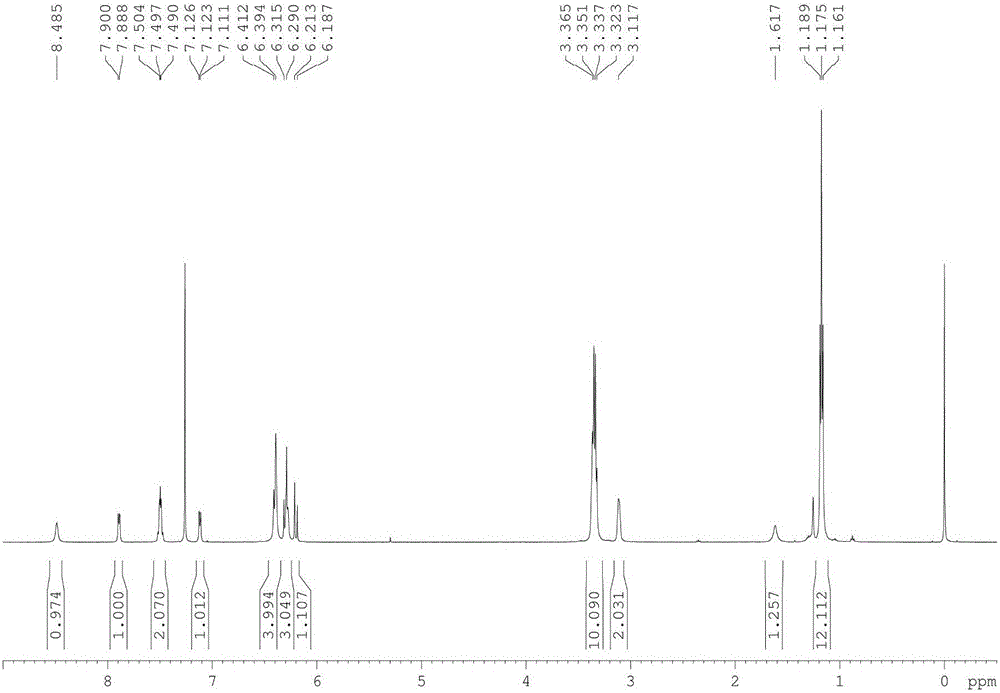
[0048] Compound 1 (97mg, 0.2mmol) was dissolved in chloroform (2ml), maleic anhydride (78mg, 0.4mmol), and heated to reflux for 10h. After the reaction was completed, the solvent was removed under reduced pressure, extracted, and finally a light red solid (130 mg, 91%) was obtained after column separation. Compound 2 1 H NMR, 13 C NMR respectively as image 3 , Figure 4 shown.
[0049] 3. Synthesis of Target ...
Embodiment 2
[0052] UV selective performance test
[0053] The target compound has good solubility in methanol. After verification, the target compound can be dissolved in 30% acetonitrile in Tris-HCl (1.0mM, pH=7.4) solvent, prepare 500ml of this solution as a stock solution (pH=7.4) .
[0054] Precisely configure the target compound as 1 x 10 -3 mol / L in 30% MeCN aqueous solution, CdCl 2 2.5H 2 O, CuCl 2 2H 2 O,AlCl 3 ,KCl,FeCl 3 ·6H 2 O,PbCl 2 ,AgNO 3 ,HgCl 2 ,NiCl 2 ·6H 2 O,MgCl 2 ·6H 2 O, NaCl, ZnCl 2 ,CrCl 3 ·6H 2 O,Ba(NO 3 ) 2 , CuCl, LiCl·H 2 O,MnCl 2 4H 2 O,CoCl 2 ·6H 2 O,CaCl 2 Isoconcentration is 5×10 -3 mol / L aqueous solution, and dissolved with 30% acetonitrile in Tris-HCl (1.0mM, pH=7.4) solution.
[0055] UV selectivity experiments such as Figure 7 As shown, take 3ml of the stock solution and place it in the liquid pool, add 30uL of the target compound, measure its initial absorbance, then add 30uL of various prepared cations respectively, and me...
Embodiment 3
[0057] Fluorescent selective performance test
[0058] The target compound has good solubility in methanol. After verification, the target compound can be dissolved in 30% acetonitrile in Tris-HCl (1.0mM, pH=7.4) solvent, prepare 500ml of this solution as a stock solution (pH=7.4) .
[0059] Precisely configure the target compound as 1 x 10 -3 mol / L in 30% MeCN aqueous solution, CdCl 2 2.5H 2 O, CuCl 2 2H 2 O,AlCl 3 ,KCl,FeCl 3 ·6H 2 O,PbCl 2 ,AgNO 3 ,HgCl 2 ,NiCl 2 ·6H 2 O,MgCl 2 ·6H 2 O, NaCl, ZnCl 2 ,CrCl 3 ·6H 2 O,Ba(NO 3 ) 2 , CuCl, LiCl·H 2 O,MnCl 2 4H 2 O,CoCl 2 ·6H 2 O,CaCl 2 Isoconcentration is 5×10 -3 mol / L aqueous solution, and dissolved with 30% acetonitrile in Tris-HCl (1.0mM, pH=7.4) solution.
[0060] Fluorescent selectivity experiments such as Figure 8 As shown, take 3ml of the stock solution and place it in the liquid pool, add 30uL of the target compound, measure its initial fluorescence intensity value, then add 30uL of various p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com