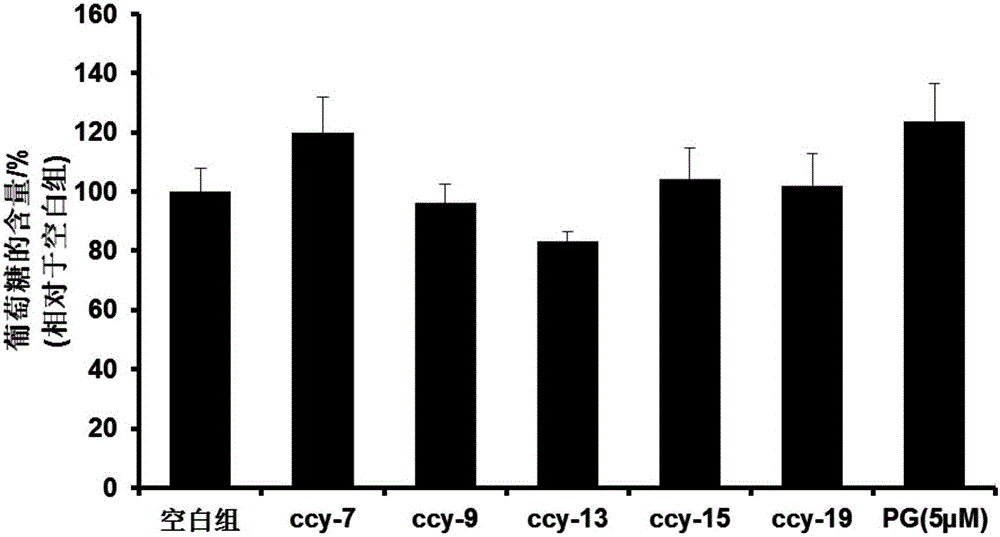
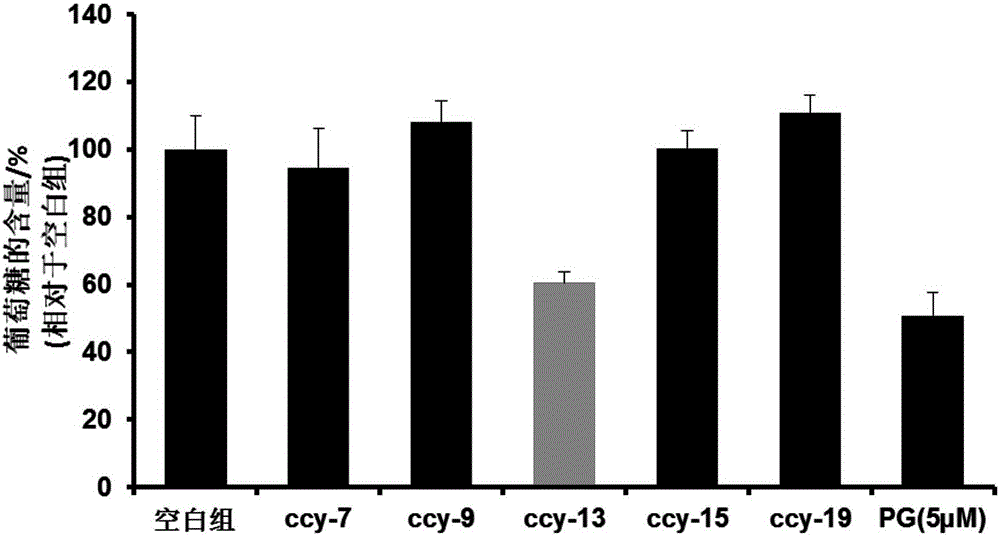
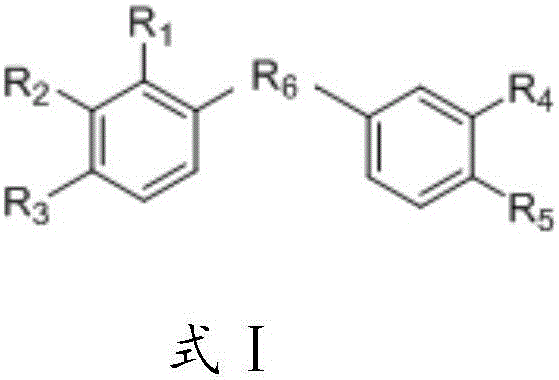
Compound, and preparation method and application thereof
A compound and reaction technology, applied in the field of compounds and their preparation, can solve the problems of low yield, difficult synthesis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0111] The processing steps of its synthetic method are:
[0112] Dissolve 2mmol of 3,4-dimethoxyacetophenone in 10mL of methanol, add 0.2mL of 50% KOH aqueous solution, heat at 70°C, dissolve 1mmol of p-benzaldehyde in 10mL of methanol, then add dropwise 3,4-dimethyl oxyacetophenone solution. After reacting for 2 h, stop heating, pour the reaction solution into water, and remove methanol by rotary evaporation, CH 2 Cl 2 Extraction, CH 3 COOCH 2 CH 3 :CH 2 Cl 2 =1:100 ccy-6 was obtained by column chromatography. Ice-water bath, under the protection of nitrogen atmosphere, inject 5ml 1mol / L BBr into the two-necked bottle 3 CH 2 Cl 2 solution, 0.3 mmol ccy-6 dissolved in 15 ml dry CH 2 Cl 2 and dropwise into BBr 3 In solution, ccy-6 and BBr 3 The molar ratio is 1 / 17. After reacting at 0-35°C for 24-48 hours, the solution is poured into ice water, stirred while pouring, and the solid is filtered, dried, and washed with methanol to obtain compound ccy-7.
[0113] Th...
Embodiment 1
[0142] Embodiment 1 compound ccy-7 and its synthesis
[0143] Dissolve 364mg (2mmol) of 3,4-dimethoxyacetophenone in 3mL of methanol, add 0.2Ml 50% KOH aqueous solution, heat at 70°C, dissolve (134mg) 1mmol of p-benzaldehyde in 4mL of methanol and add dropwise 3 , 4-dimethoxyacetophenone solution. After reacting for 2 h, stop heating, pour the reaction solution into water, and remove methanol by rotary evaporation, CH 2 Cl 2 Extraction, CH 3 COOCH 2 CH 3 :CH 2 Cl 2 =1:10~1:20 Column chromatography separation obtained 353mg ccy-6, yield 77%. Ice-water bath, nitrogen atmosphere protection, inject 5ml 1mol / L BBr into a 100ml two-necked bottle 3 CH 2 Cl 2 solution, 137mg (0.3mmol) ccy-6 dissolved in 15ml dry CH 2 Cl 2 and dropwise into BBr 3 In solution, ccy-6 and BBr 3 The molar ratio was 1 / 17. After reacting at 25°C±5°C for 24h, the solution was poured into ice water, stirred while pouring, filtered to obtain a solid, and the filter residue was dried, and column ch...
Embodiment 2
[0147] Embodiment 2 compound ccy-9 and its synthesis
[0148] 339 mg (2 mmol) of 3,4-dimethoxybenzaldehyde and 160 mg (1 mmol) of m-benzophenone were reacted to obtain 185 mg of ccy-8 with a yield of 41%. 188mg (0.4mmol) ccy-8 and 4.5ml 1mol / L BBr 3 CH 2 Cl 2 Solution reaction, ccy-8 and BBr3 The molar ratio of ccy-9 is 1 / 11, and the reaction conditions and synthesis method are the same as in Example 1 to obtain 100 mg ccy-9 with a yield of 61%. The product was tested by IR spectrum and 1 H NMR spectrum measurement, analysis result is as follows:
[0149] IR spectrum (KBr coating cm- 1 ): 3448,1654,1597,1514,1437,1367,1284,1167,1103,1061,978,790,678,567.
[0150] 1 H NMR Spectrum (ppm): (300MHz, DMSO) δ9.74(s, 2H), 9.13(s, 2H), 8.61(s, 1H), 8.32(dd, J=7.8, 1.5Hz, 2H), 7.71 (t,J=7.8Hz,1H),7.65(s,4H),7.28(d,J=1.8Hz,2H),7.19(dd,J=8.4,2.1Hz,2H),6.80(d,J= 8.1Hz,2H),6.80(d,J=8.1Hz,2H).
[0151] (solvent DMSO-d 6 , TMS internal standard)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Decomposition temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com