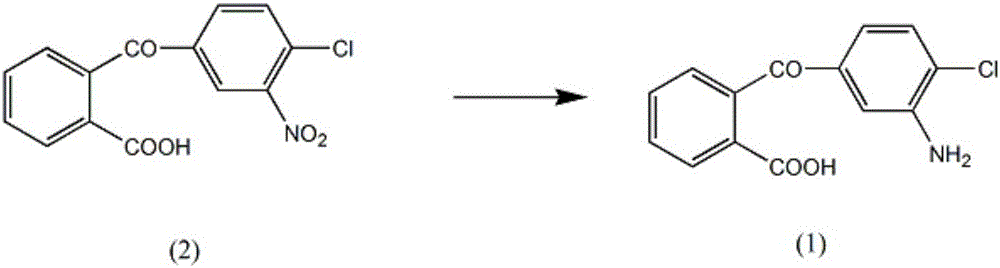
Synthesis method of chlorthalidone medicine intermediate 2-(3-amino-4-chlorobenzoyl)benzoic acid
A technology of chlorobenzoyl and synthetic methods, which is applied in the field of organic synthesis, can solve the problems such as the need to improve the reaction yield and long reaction time, and achieve the effects of shortening the reaction time, increasing the reaction yield, and improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A kind of synthetic method of chlorthalidone medicine intermediate 2-(3-amino-4-chlorobenzoyl)benzoic acid, carries out according to the following steps:
[0021] A, add 2-(3-nitro-4-chlorobenzoyl) benzoic acid (2) 0.23mol in reaction vessel, mass fraction is 35% sodium nitrate solution 300ml, mass fraction is 24% potassium carbonate solution 500ml, Raise the solution temperature to 60°C, add 1.3 L of 2-amino-5-chlorobenzoic acid solution with a mass fraction of 42%, 0.31 mol of cuprous chloride, 0.2 mol of potassium iodide, control the stirring speed at 160 rpm, and raise the solution temperature to 70 ℃, reflux for 3h;
[0022] B. Lower the solution temperature to 20°C, filter, wash the filter cake with a 52% sodium bisulfite solution in mass fraction, combine the washing liquid, raise the solution temperature to 50°C, add 300ml of ammonium chloride with a mass fraction of 37% Solution, fully stirred for 90min;
[0023] C. Reduce the solution temperature to 10°C, fi...
Embodiment 2
[0025] A kind of synthetic method of chlorthalidone medicine intermediate 2-(3-amino-4-chlorobenzoyl)benzoic acid, carries out according to the following steps:
[0026] A, add 2-(3-nitro-4-chlorobenzoyl) benzoic acid (2) 0.23mol in reaction vessel, mass fraction is 33% sodium nitrate solution 300ml, mass fraction is 24% potassium carbonate solution 500ml, Raise the solution temperature to 63°C, add 1.3 L of 2-amino-5-chlorobenzoic acid solution with a mass fraction of 42%, 0.31 mol of cuprous chloride, and 0.2 mol of potassium iodide, control the stirring speed at 170 rpm, and raise the solution temperature to 73 ℃, reflux for 3h;
[0027] B. Reduce the solution temperature to 23°C, filter, wash the filter cake with a 53% sodium bisulfite solution, combine the washing liquid, raise the solution temperature to 52°C, add 300ml of ammonium chloride with a mass fraction of 37% Solution, fully stirred for 110min;
[0028] C. Reduce the temperature of the solution to 13°C, filter...
Embodiment 3
[0030] A kind of synthetic method of chlorthalidone medicine intermediate 2-(3-amino-4-chlorobenzoyl)benzoic acid, carries out according to the following steps:
[0031] A, add 2-(3-nitro-4-chlorobenzoyl) benzoic acid (2) 0.23mol in reaction vessel, mass fraction is 37% sodium nitrate solution 300ml, mass fraction is 26% potassium carbonate solution 500ml, Raise the temperature of the solution to 65°C, add 1.3L of 2-amino-5-chlorobenzoic acid solution with a mass fraction of 45%, 0.31mol of cuprous chloride, and 0.2mol of potassium iodide, control the stirring speed at 190rpm, and raise the solution temperature to 76°C , reflux for 4h;
[0032] B, reduce solution temperature to 26 ℃, filter, filter cake is that 56% sodium bisulfite solution washes with mass fraction, merges washing liquid, raises solution temperature to 55 ℃, adds 300ml mass fraction and is 39% ammonium chloride solution, Fully stir for 130min;
[0033] C. Reduce the temperature of the solution to 15°C, filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com