Preparation method and application of parthenolide analog
A kind of technology of parthenolide and analogs, which is applied in the preparation of parthenolide analogs and the application field of preparing anticancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
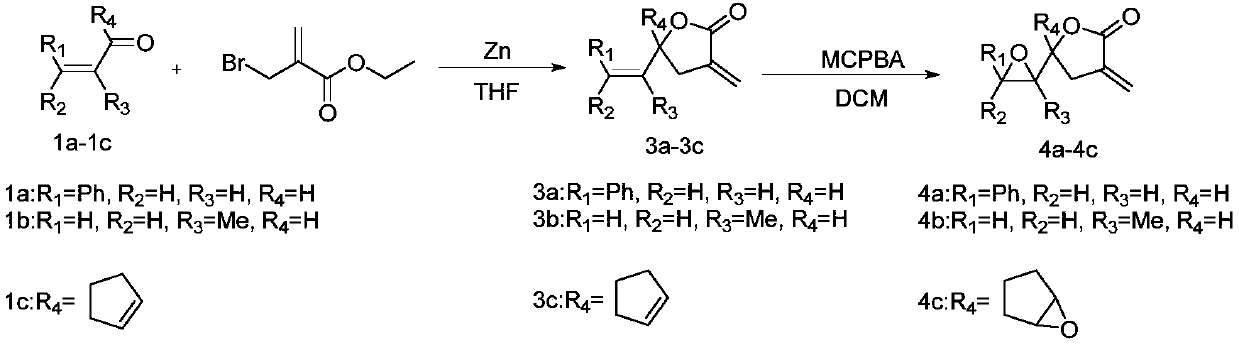
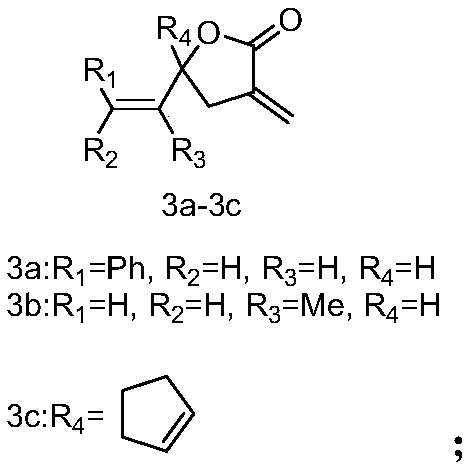
[0027] The synthesis of embodiment 1 compound 3a-3c
[0028]
[0029] Weigh Zn powder (2.0mmol) in a 100mL round bottom flask, add 5mL tetrahydrofuran, slowly add 2-bromoethyl methacrylate (2.0mmol), N 2 For replacement protection, the reaction solution was heated with a heat gun and ultrasonicated until the Zn powder was completely dissolved, then aldehyde (1.0 mmol) was slowly added dropwise, and the reaction was carried out at room temperature for 10-15 minutes (monitoring the reaction progress by TLC). After the reaction, THF was removed by rotary evaporation under reduced pressure, quenched by adding water, extracted three times with ethyl acetate, collected the organic phase, washed three times with saturated brine, dried with anhydrous sodium sulfate, and the concentrated column layer Analysis and purification [eluent: V (petroleum ether): V (ethyl acetate) = 10:1-5:1] to obtain target compounds 3a-3c.
[0030] Compound 3a [eluent: V (petroleum ether): V (ethyl acet...
Embodiment 2
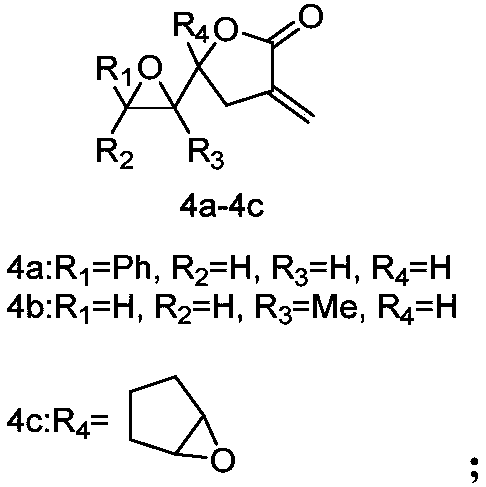
[0035] The synthesis of embodiment 2 compound 4a-4c
[0036]
[0037] The lactone compound (1.0 mmol) was weighed and dissolved in dichloromethane, placed in an ice bath, the compound MCPBA (1.5 mmol) was weighed and added to the reaction solution, and the reaction was carried out for 30 min and moved to room temperature (reaction progress was monitored by TLC). After the reaction, wash the organic phase 3 times with 1M NaOH aqueous solution, collect the organic phase, and wash the organic phase with anhydrous NaSO 4 After drying, the concentrated product was rotary evaporated under reduced pressure and purified by column chromatography [eluent: V (petroleum ether): V (ethyl acetate) = 10:1-5:1] to obtain compounds 4a-4c.
[0038]
[0039] Compound 4a [eluent: V (petroleum ether): V (ethyl acetate) = 10:1-4:1]: white solid, melting point 59.8°C, yield 80%; 1 H NMR (300MHz, CDCl 3 )δ7.31-7.16(m,5H),6.22-6.20(t,J=3.0Hz,1H),5.65-5.63(t,J=3.0Hz,1H),4.60-4.54(m,1H),3.74 -3...
Embodiment 3
[0044] The synthesis of embodiment 3 compound 5a-5h
[0045]
[0046] NaOH (1.0mmol) and H 2 o 2 (1.0mmol) was slowly added to a round-bottomed flask, 10mL MeOH was added, and then substrate aldehyde or ketone (1.0mmol) was added, and reacted at room temperature for 10min (reaction progress was monitored by TLC). Purified by column chromatography [V (petroleum ether): V (ethyl acetate) = 10:1 ~ 5:1] to obtain compounds 5a-5f.
[0047]
[0048] Compound 5a [eluent: V (petroleum ether): V (ethyl acetate) = 8:1-5:1]: white solid, yield 80%; 1 HNMR (300MHz, CDCl 3 )δ7.31-7.39 (m, 5H), 3.94-3.93 (d, J = 3.0Hz, 1H), 3.43-3.42 (d, J = 3.0Hz, 1H), 2.13 (s, 3H); 13 C NMR (75MHz, CDCl 3 )δ203.2, 134.0, 128.0, 127.7, 124.7, 62.4, 56.7, 23.8
[0049]
[0050] Compound 5b [eluent: V (petroleum ether): V (ethyl acetate) = 10:1-5:1]: white solid, yield 85%; 1 HNMR (300MHz, CDCl 3 )δ7.93-7.90(m,2H),7.56-7.50(m,1H),7.42-7.37(m,2H),7.42-7.26(m,5H),4.22-4.21(d,J=3.0Hz, 1H), 3.9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com