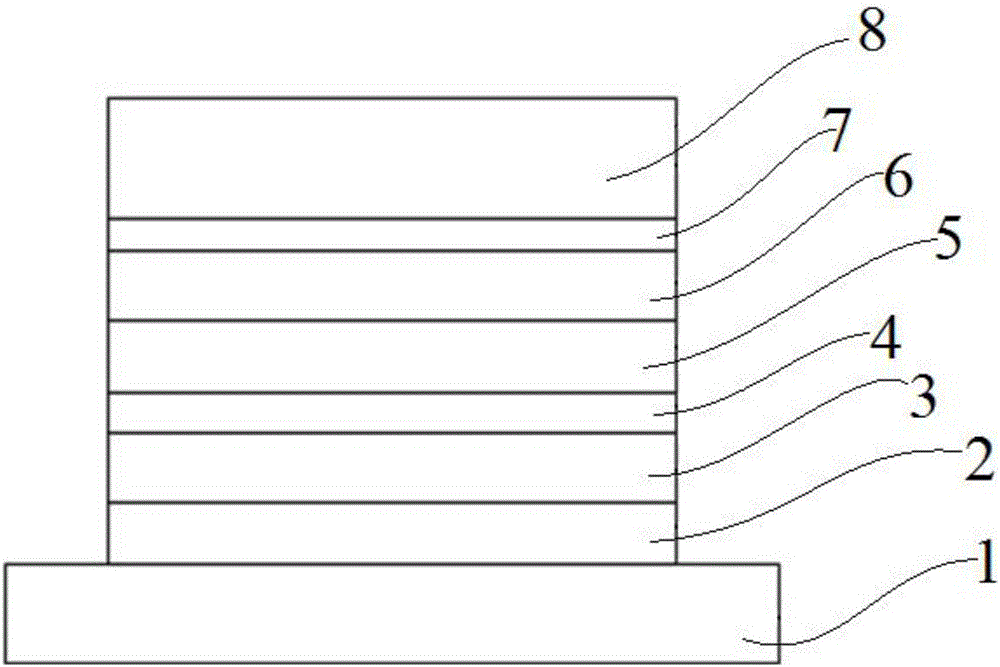
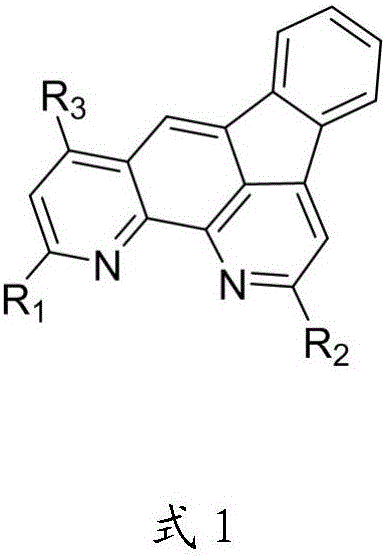
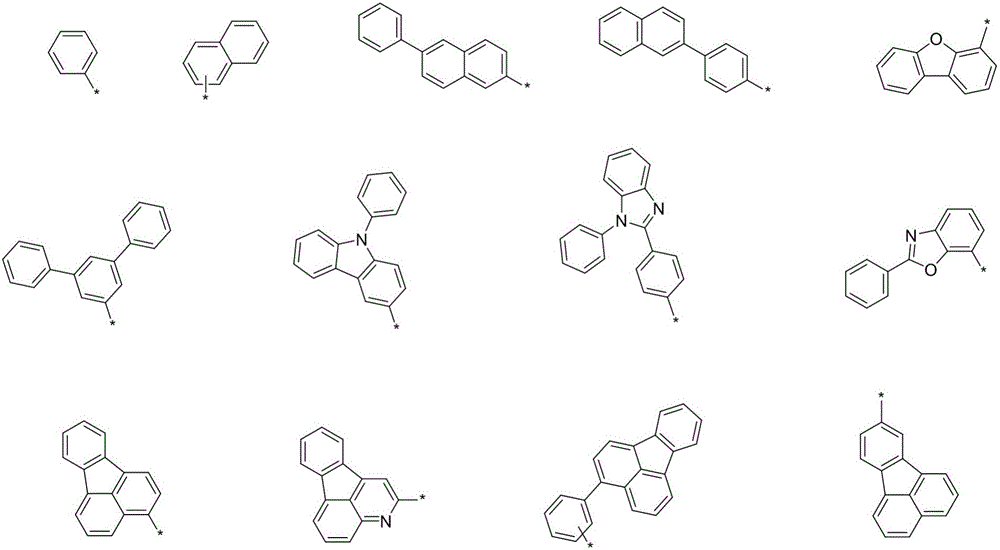
Organic optoelectronic material with indeno-phenanthroline structure and preparing method and application thereof
An organic photoelectric material, indenophenanthroline technology, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of rare electron transport materials, achieve good industrialization prospects, high glass transition Effect of temperature and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Preparation of the aforementioned compound E01
[0074] Preparation of intermediate one (a):
[0075]
[0076] At -78°C and under the protection of nitrogen, take 67.6g of 4,7-dibromophenanthroline (200.0mmol) and disperse it in 500g of tetrahydrofuran, then add dropwise 176mL of 2.5mol / L containing n-butyl Lithium n-hexane solution, after the dropwise addition, keep it warm for 2.0 hours, then add 33.0g of trimethylchlorosilane dropwise at -78°C, keep it warm for 2.0 hours after the drop, and then slowly warm the reaction system to room temperature , then poured the system into 200g of water, stirred and reacted for 30min, then added 500g of ethyl acetate for extraction, the system was layered, washed with water, and finally the organic phase was desolvated under reduced pressure until there was no fraction, and 64.7g of intermediate- (a), without further purification.
[0077] Preparation of intermediate one (b):
[0078]
[0079] At -78°C and unde...
Embodiment 2
[0099] Example 2: Preparation of the aforementioned compound E26
[0100] The preparation of intermediate two (a):
[0101]
[0102] At -78°C and under the protection of nitrogen, 52.1 g of 4-bromodibenzofuran (211.0 mmol) was dispersed in 400 g of tetrahydrofuran, and then 93 mL of n-butyllithium containing 2.5 mol / L was added dropwise. After the addition of the n-hexane solution is complete, keep it warm for 2.0 hours, and then slowly raise the temperature to -10°C for storage until use.
[0103] Take 32.5g of intermediate one (c) (100.0mmol) and dissolve it in 300g of tetrahydrofuran, add the above-mentioned phenyllithium solution dropwise at -10°C, keep the reaction for 2.0 hours after dropping, and then slowly raise the temperature of the reaction system to room temperature , and then poured the system into 200g of water, stirred and reacted for 30min, then added 500g of dichloromethane for extraction, the system was layered, washed with water, and dried over anhydrou...
Embodiment 3
[0122] Example 3: Preparation of the aforementioned compound E38
[0123] Preparation of intermediate three (a):
[0124]
[0125] At -78°C and under the protection of nitrogen, take 16.6g of bromobenzene (105.0mmol) and disperse it in 150g of tetrahydrofuran, then add dropwise 47mL of n-hexane solution containing n-butyllithium with a concentration of 2.5mol / L, dropwise After the addition, keep the reaction for 2.0 hours, and then slowly raise the temperature to -10°C for storage until use.
[0126] Take 24.5g of intermediate bis(a) (50.0mmol) and dissolve it in 120g of tetrahydrofuran, add the above-mentioned phenyllithium solution dropwise at -10°C, keep the reaction for 2.0 hours after dropping, and then slowly raise the temperature of the reaction system to room temperature , and then poured the system into 150g of water, stirred and reacted for 30min, then added 300g of dichloromethane for extraction, the system was layered, washed with water, and dried over anhydrou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com