Indian kalimeris herb active component and compounds as well as preparation and application
A technology for active components and flavonoid glycoside compounds, applied in the field of medicine, can solve problems such as serious drug resistance, environmental pollution, etc., and achieve the effects of simple preparation method and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 The preparation method of Malan water-soluble active components and two flavonoid glycoside compounds kaempferol-3-O-rutinoside and isorhamnetin-3-O-rutinoside
[0024] (1) Crushing and extraction:
[0025] Take 1 kg of dried Malan and grind it, extract it twice with 10L methanol (industrial grade) at room temperature, and concentrate it by suction filtration for 1 day each time to obtain a crude sample of the methanol extract, extract and separate it with ethyl acetate and water, and let it stand , after concentrating the aqueous layer under reduced pressure and spinning to dryness, 28 g of a crude sample of the aqueous layer of B was obtained.
[0026] (2) Separation and purification:
[0027] The crude sample of the aqueous layer is first separated by an ODS open column (the solvent system is methanol: water = 3:7, 5:5, 6:4, 7:3, 8:2, 9:1, 10:0 by volume) The active sample is separated with a silica gel open column (200-300 mesh, the following solvent sys...
Embodiment 1
[0029] Qualitative identification of the physical and chemical characteristics and chemical structure of the compound kaempferol-3-O-rutinoside and isorhamnetin-3-O-rutinoside obtained in Example 1:
[0030] The structures of compounds kaempferol-3-O-rutinoside and isorhamnetin-3-O-rutinoside have been 1 H NMR, MS and literature comparison confirmed
[0031] Physicochemical properties of compound kaempferol-3-O-rutinoside: yellow solid, Molecular formula is C 27 h 30 o 15 ; ESI-TOF-MS: m / z 595.1663 (M+H) + , hydrogen spectrum data: 1 H NMR (500MHz, CD 3 OD): δ=1.12(3H,d,J=6.0Hz,H-6"'),3.25-2.81(10H,m,H-2"-6" / 2"'-5"'),4.51( 1H, s, H-1"'), 5.12 (1H, d, J=7.5Hz, H-1"), 6.89 (2H, d, J=8.5Hz, H-3' / 5'), 8.07( 2H,d,J=8.5Hz,H-2' / 6'), 6.41(1H,s,H-8), 6.21(1H,s,H-6).
[0032] Physicochemical properties of compound isorhamnetin-3-O-rutinoside: yellow solid, Molecular formula is C 28 h 32 o 16 ,ESI-TOF-MS:m / z 625.1765(M+H) + , hydrogen spectral data: 1 H NMR (500MHz, CD ...
Embodiment 3
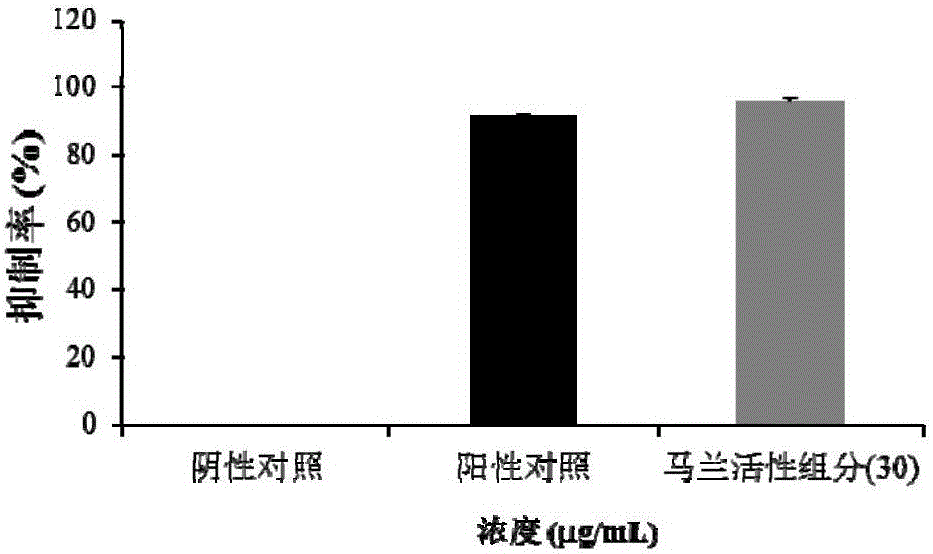
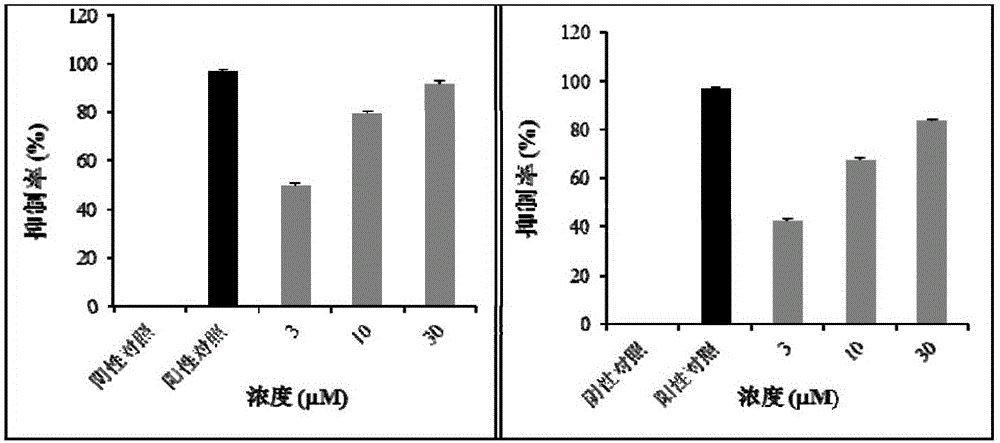
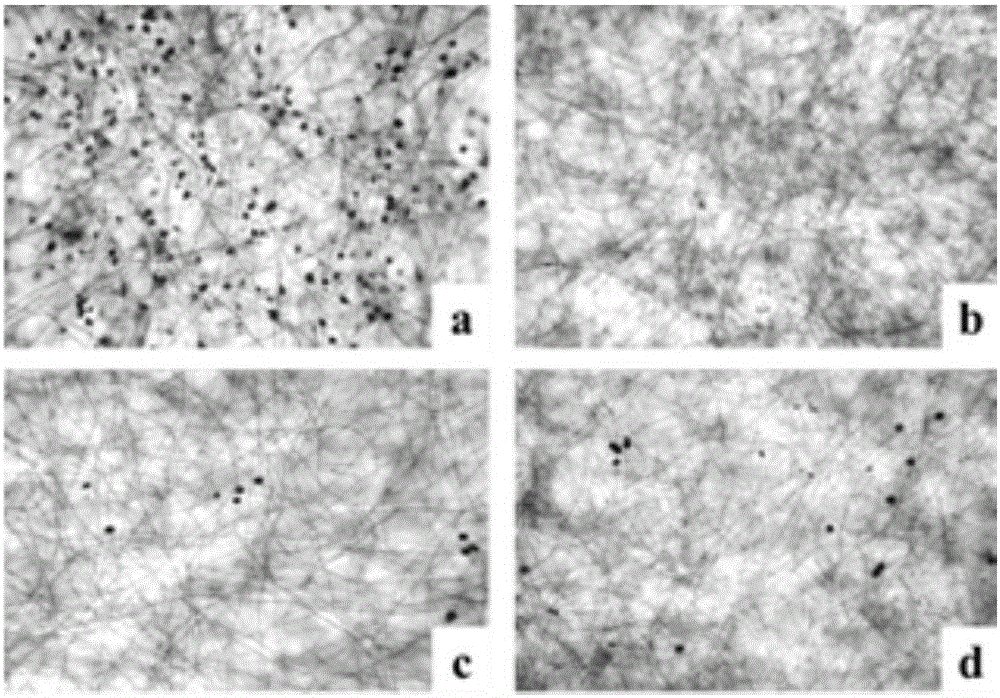
[0034] The water-soluble active components and compounds of Malan can significantly inhibit the production of Phytophthora sporangia in the screening model of Phytophthora capsici.
[0035] At present, the research on active substances against Phytophthora mainly focuses on inhibiting the growth of mycelia and germination of zoospores. There are very few studies on active substances that can inhibit the production of sporangia in the asexual reproduction stage of Phytophthora. Metalaxyl is currently the most widely used pesticide in the prevention and treatment of plant diseases. It can strongly inhibit the growth of Phytophthora mycelium and the production of asexual sporangia. The present invention finds that the water-soluble active component of Malan and its two flavonoid glycoside compounds, kaempferol-3-O-rutinoside and isorhamnetin-3-O-rutinoside, can significantly inhibit the production of Phytophthora sporangia in the Phytophthora capsici model.
[0036] 1. Experiment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com