Animal derived serum-free cell freezing medium
A technology of animal source and cryopreservation solution, applied in the field of cell therapy, can solve the problems of increasing animal pathogens, pollution, virus transmission, etc., and achieve the effects of low cost, reducing animal pathogen pollution, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Cell culture (taking cell CIK as an example)
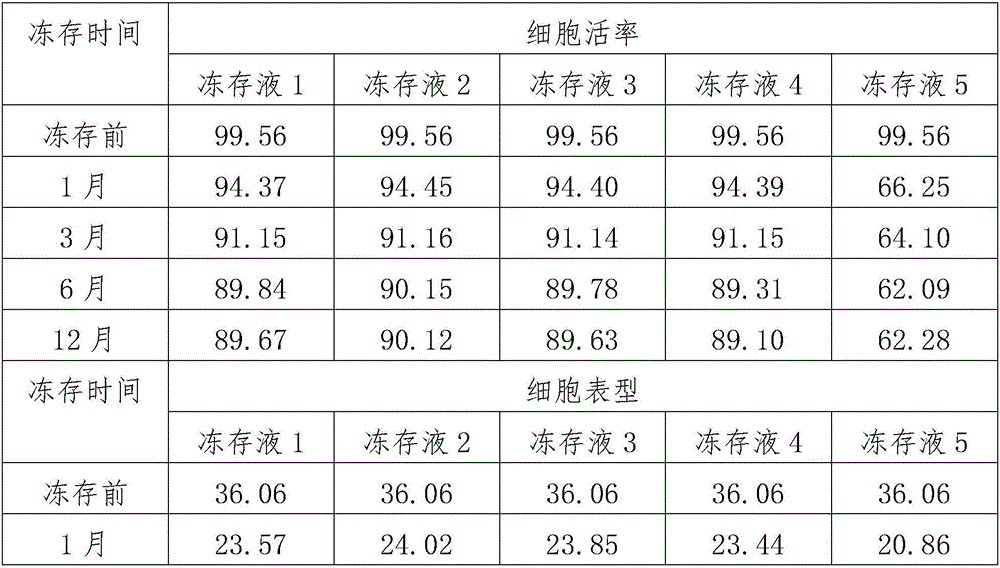
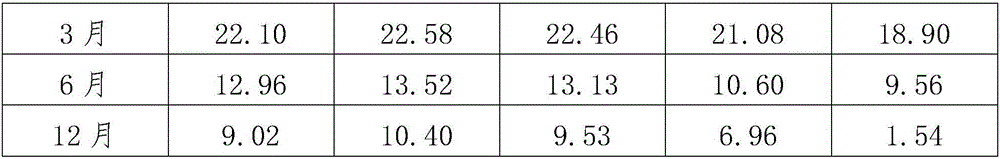
[0018] Use lymphocyte separation medium density gradient centrifugation to separate peripheral blood mononuclear cells, or to revive frozen mononuclear cells. Centrifuge at 500 g of normal saline for 5 minutes, and remove the supernatant. Count the cells and make 1*106 / ml cell suspension with the corresponding culture medium. Add 2ml of culture medium and cell suspension into a 6-well plate, with 3 parallel wells in each group. On the first day, 1000IU / ml of INF-r was added and cultured in a carbon dioxide incubator. After 24 hours, 50ng / ml of CD3 monoclonal antibody and 500IU / ml of IL-2 were added. Afterwards, every 48 hours, add corresponding culture medium and 500IU / ml IL-2, and count to 1*106 / ml cell concentration. The cell morphology was observed after co-cultivation for 14 days, and the phenotype and viability of CIK cells were detected at 14 days. The specific test results are shown in Table 1.
Embodiment 2
[0020] According to the volume ratio, 5% HSA was mixed with 85% HES and 10% DMSO to prepare cell cryopreservation solution 1, which was stored at 4°C for later use.
[0021] The CIK cells expanded in Example 1 were treated with a concentration of 5×10 7 The concentration per ml was suspended in the above-mentioned cryopreservation solution 1, placed in a programmed cooling box and placed in a -80°C ultra-low temperature refrigerator overnight, and transferred to liquid nitrogen for cryopreservation the next day.
[0022] After 1 month, 3 months, 6 months and 1 year after cryopreservation, the cells were recovered in a 37°C water bath. After 5 days of culture, the cell phenotype and viability were detected. The specific test results are shown in Table 1.
Embodiment 3
[0024] Mix 10% HSA with 80% HES and 10% DMSO according to the volume ratio to prepare cell cryopreservation solution 1, and store it at 4°C for later use.
[0025] The CIK cells expanded in Example 1 were treated with a concentration of 5×10 7 The concentration per ml was suspended in the above-mentioned cryopreservation solution 2, placed in a programmed cooling box and placed in a -80°C ultra-low temperature refrigerator overnight, and transferred to liquid nitrogen for cryopreservation the next day.
[0026] After 1 month, 3 months, 6 months and 1 year after cryopreservation, the cells were recovered in a 37°C water bath. After 5 days of culture, the cell phenotype and viability were detected. The specific test results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com