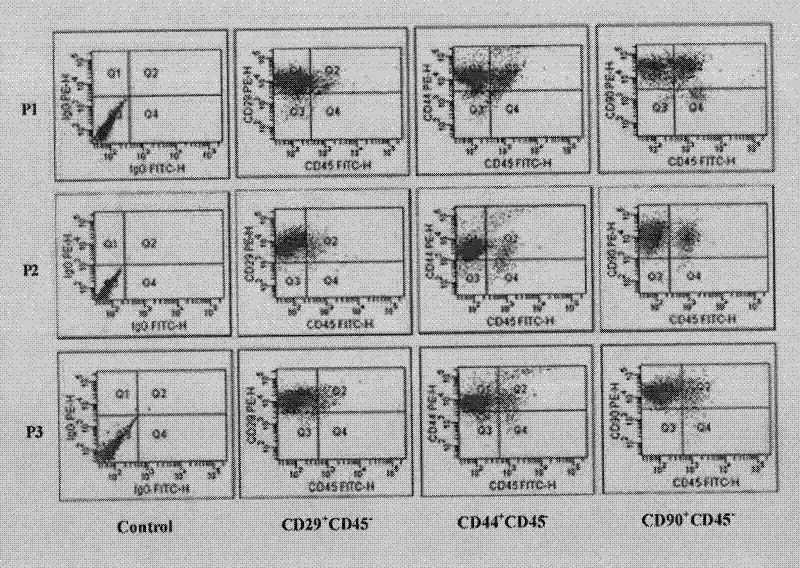
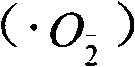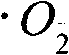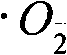Patents
Literature
42results about How to "Maintain biological function" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Low-expression CYP7A1 hepatic cell and constructing method thereof
InactiveCN101591653AImprove microenvironmentImprove the quality of lifeFermentationVector-based foreign material introductionArtificial liverVector system
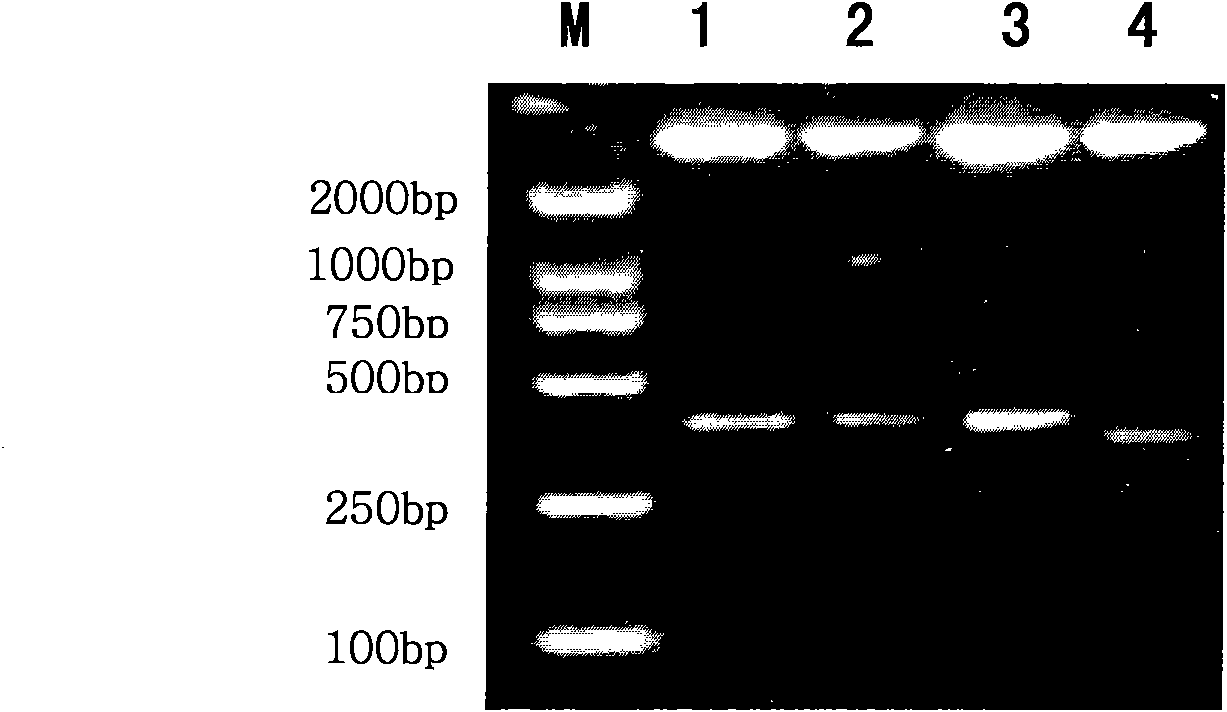
The invention discloses a low-expression CYP7A1 hepatic cell and a constructing method thereof. A method for inhibiting the CYP7A1 expression level of the hepatic cell comprises the step of introducing an encoding gene of small-interference RNA which is used for inhibiting the CYP7A1 gene expression into the hepatic cell so as to inhibit the CYP7A1 expression level of the hepatic cell. A slow virus vector system can be used for introducing the encoding gene of small-interference RNA which is used for inhibiting the CYP7A1 gene expression into the hepatic cell. An experiment proves that the method of the invention can obviously regulate the expression level of the CYP7A1 gene of the hepatic cell in an mRNA level and a protein level downwards and can further effectively reduce the secretoryvolume of total bile acid. The Low-expression CYP7A1 hepatic cell and the constructing method thereof can be used for the fundamental research and the clinical application of biologic artificial liver supporting treatment and have a wide application prospect.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Low-temperature preserved solid culture medium of skin model and preservation method of low-temperature preserved solid culture medium
ActiveCN104222068ANormal physiological environmentNormal growth environmentDead animal preservationCulture fluidCuticle
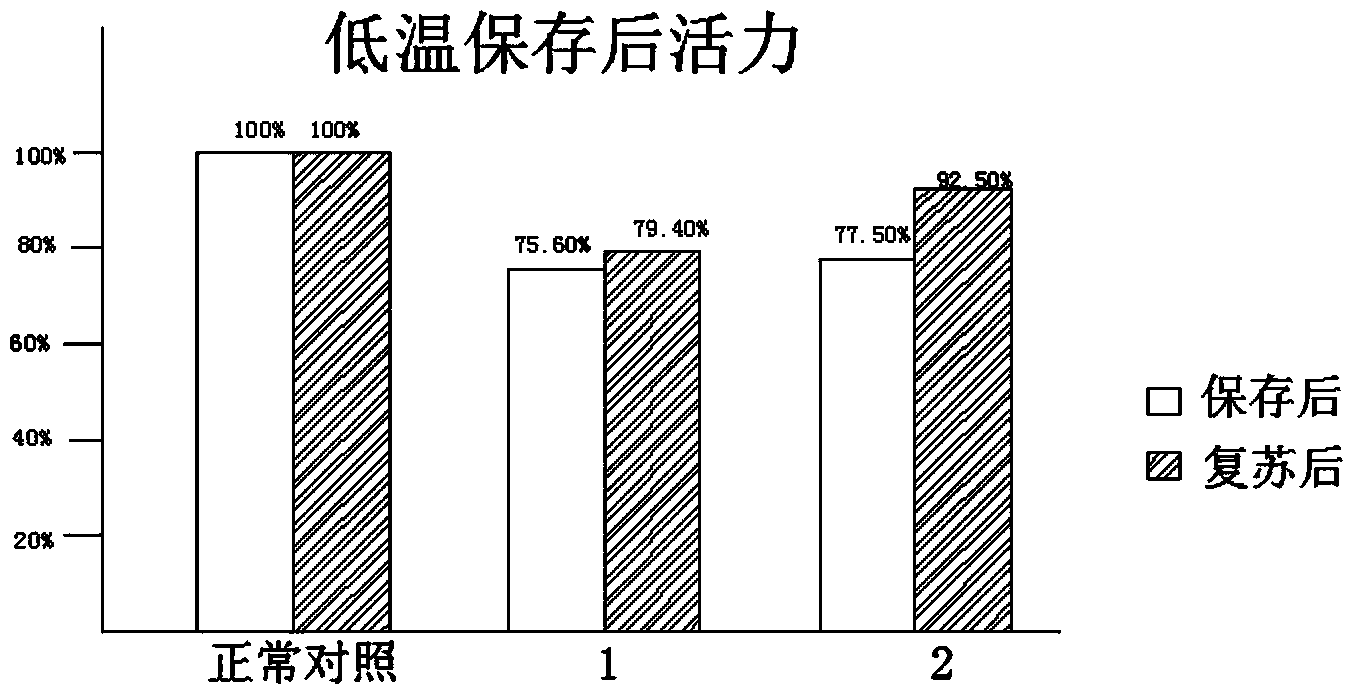
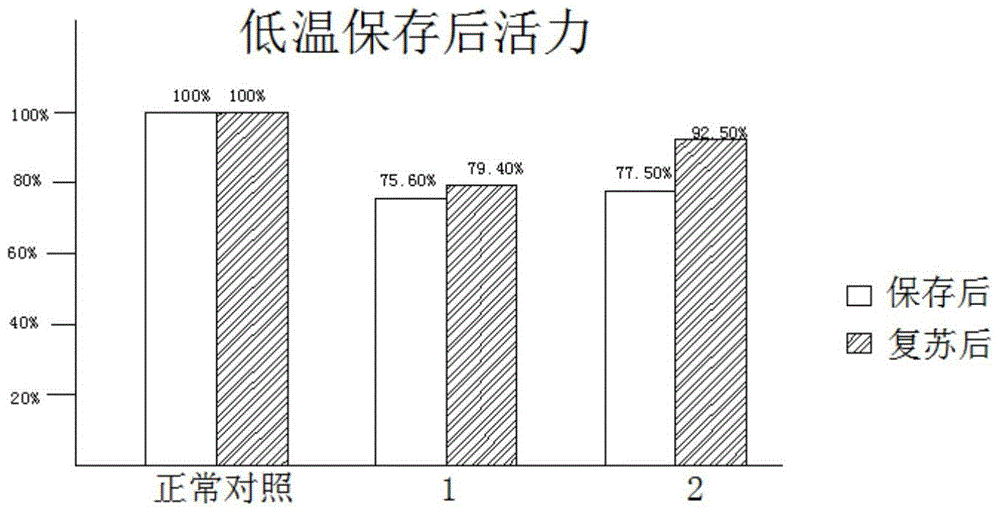
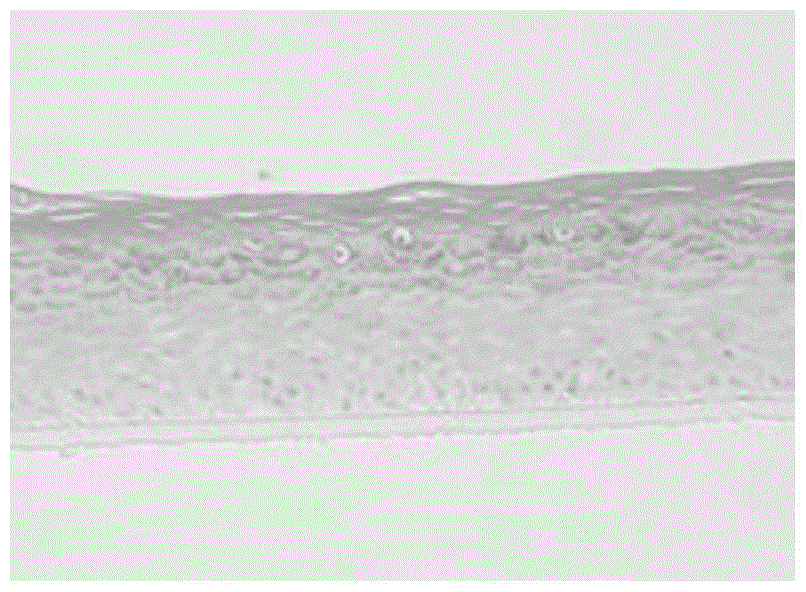
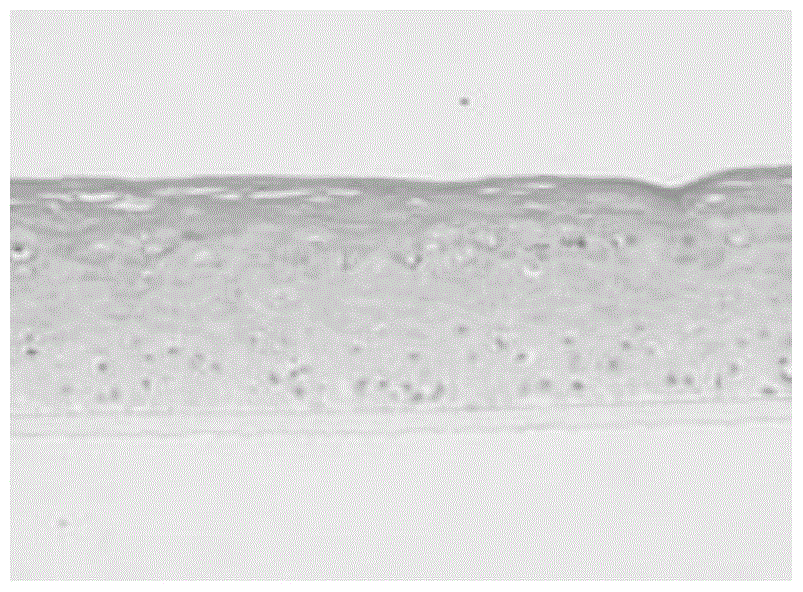
The invention discloses a low-temperature preserved solid culture medium used for a tissue engineering skin model and a preservation method of the low-temperature preserved solid culture medium. The low-temperature preserved solid culture medium is prepared by the following steps: adding a low-temperature protection agent into a common culture medium for epidermis cells to prepare a basic culture solution; and mixing the basic culture solution with agarose gel and condensing to form the solid culture medium suitable for the skin model. The tissue engineering skin model is embedded into the culture medium, is preserved at 4-8 DEG C for 24-72 hours, and then is resuscitated by a common epidermis cell culture solution. The solid culture medium can be used for reducing tissue activity reduction and structure damages of cells and skin tissues, caused by a low-temperature preservation condition, to the greatest extent.
Owner:GUANGDONG BOXI BIO TECH CO LTD
Compound active amnion material, preparation method and application thereof and compound active amnion uterine cavity repair stent
ActiveCN103349798AStructure remains intactMaintain biological functionSurgeryMedical devicesSacculeMedicine
The invention provides a compound active amnion material, a preparation method and application thereof and a compound active amnion uterine cavity repair stent. The preparation method comprises the following steps: (1) providing a collagen sponge prepare liquid or a compound collagen sponge prepare liquid; (2) providing an amnion with both an epithelial layer and a basement membrane; (3) pouring and coating the prepared prepare liquid on the basement membrane of the amnion to enable the basement membrane and the prepare liquid to be subject to crosslinking, and then preparing the compound active amnion material through freeze drying. The compound active amnion material not only keeps the complete structure and the due biological function of the amnion but also endow the amnion with a certain rigidity, so that the compound active amnion material has stronger maneuverability in application after TCRA (Transcervical Resection Of Adhesions) operation; the compound active amnion uterine cavity repair stent integrates the compound active amnion material and a saccule at the front end of a pipe body, the saccule expands under the action of an external force to push out the compound active amnion material, and then the compound active amnion material is successfully attached to the inner wall of the uterine cavity; the stent is convenient to take out and prevents secondary damage.
Owner:JIANGXI RUIJI BIOTECH CO LTD
Method for preparing porcine hepatocyte and mesenchymal stem cell co-microencapsulated internal bio-artificial liver
InactiveCN102218161AProlong survival timePromote proliferation and differentiationHaemofiltrationArtificial cell constructsArtificial liverTherapeutic effect
The invention relates to a method for preparing a porcine hepatocyte and mesenchymal stem cell co-microencapsulated internal bio-artificial liver. The invention comprises the following steps: establishing an in-vitro culture amplification system for porcine mesenchymal stem cells, performing in-situ perfusion separation of porcine primary hepatocytes by a two-step collagenase method, and preparing alginate-poly-L-lysine microencapsulated hepatocytes and mesenchymal stem cells. The invention overcomes the defects that internal cells usually can not survive for a long time; the proliferation ability is quite limited; and the function of the injected hepatocytes for generating various stimulating factors for promoting liver regeneration is inhibited. The invention introduces mesenchymal stem cells into an internal bio-artificial liver system, fully simulates the in-vivo microenvironment of hepatocytes by heterogeneous intercellular interaction, effectively prolongs the survival time of hepatocytes, promotes the proliferation and differentiation of hepatocytes, maintains the specific biological functions of hepatocytes, and thus significantly improves the efficacy of the internal bio-artificial liver on treating hepatic failure.
Owner:THE AFFILIATED DRUM TOWER HOSPITAL MEDICAL SCHOOL OF NANJING UNIV
DMSO-free human umbilical cord mesenchymal stem cell injection cryopreservation liquid
PendingCN112772637ASo as not to damageReduce adverse effectsDead animal preservationSodium Chloride InjectionMesenchymal stem cell
The invention discloses a DMSO-free human umbilical cord mesenchymal stem cell injection cryopreservation liquid, which is characterized by being prepared from the following raw materials in parts by volume: 50 to 60 parts of compound electrolyte injection, 20 to 40 parts of dextran 40 glucose injection, 1 to 10 parts of sodium chloride injection, 1 to 10 parts of glucose injection, 30 to 50 parts of human serum albumin, and 1 to 10 parts of a mesenchymal stem cell serum-free medium. The cryopreservation liquid does not contain DMOS or serum, so that the risk of clinical use is reduced, the influence of the uncertainty of serum components and the instability of serum culture on the normal induced differentiation function of the mesenchymal stem cells is avoided, and the cryopreservation liquid enables the human umbilical cord mesenchymal stem cells to keep a good cryopreservation effect, and the human umbilical cord mesenchymal stem cells have high survival rate after cryopreservation and resuscitation. In addition, the cells cryopreserved by the cryopreservation liquid can be directly diluted and then applied clinically, components of the cryopreservation liquid do not need to be removed through centrifugation, and the cryopreservation liquid can be used as an auxiliary material and directly applied to clinical administration, so that the cryopreservation liquid is more convenient to use.
Owner:朱灏
Modified polyethylene glycol superoxide dismutase (mPEG-SOD) nanoemulsion capable of being directly externally used for skin and preparation method thereof
ActiveCN101947312AImproves transdermal penetrationImprove stabilityCosmetic preparationsPeptide/protein ingredientsExternal applicationGlycerol
The invention discloses modified polyethylene glycol superoxide dismutase (mPEG-SOD) nanoemulsion capable of being directly externally used for skin and a preparation method thereof. The mPEG-SOD nanoemulsion is prepared from the following raw materials in part by weight: 7 parts of isopropyl myristate, 30 parts of caprylocaproyl macrogolglycerides, 10 parts of polyglyceryl-3-dioleate, 50 parts of mPEG-SOD aqueous solution and 3 parts of vitamin E. The mPEG-SOD nanoemulsion solves the problem that the SOD has low stability, is easy to deactivate, and has poor penetrability for external application to the skin. The modified polyethylene glycol superoxide dismutase (mPEG-SOD) nanoemulsion capable of being directly externally used for the skin is a preparation with good stability and capacity of persistently protecting the bioactivity of the SOD, and can quickly penetrate the skin so as to greatly improve the biological effect of the SOD. The preparation method has the advantages of simple preparation process, easy actual operation, no need of special instrument and equipment, and high-efficiency and quick preparation.
Owner:董萍 +1
Serum-free medium for porcine hepatocytes and preparation method of serum-free medium
InactiveCN107043738AGood growthMaintenance of functionCulture processCell culture mediaSerum free mediaVitamin C
The invention relates to a serum-free medium for porcine hepatocytes and a preparation method of the serum-free medium. According to the serum-free medium for the porcine hepatocytes, a basic culture solution is added with the following components: 10.00mg / L of bovine insulin, 5.50mg / L of human transferrin, 5.02mg / L of sodium selenite, 2.00mg / L of ethanol amine, 196.26mug / L of dexamethasone, 0.5mg / L of fibronectin, 10.00mg / L of vitamin C, 60.00mug / L of hepatocyte growth factors, 80.00mug / L of epidermal growth factors, 2.00mg / L of glucagon and 1.50g / L of bovine serum albumin. The serum-free medium for the porcine hepatocytes is capable of being used for culturing porcine primary hepatocytes, the porcine primary hepatocytes are very equivalent to that cultured by a serum-containing medium in form and vigor and the growth condition is obviously good; the original biological characteristics and biological functions of the porcine hepatocytes can be well kept; the negative effects caused by a traditional serum-containing medium can be avoided; the condition requirements of normal growth and division and proliferation of the hepatocytes can be met.
Owner:SHAOGUAN COLLEGE
Refined manufacturing of photo-control-hybridized cross-linked degradable support and bone tissue engineering application of photo-control-hybridized cross-linked degradable support
ActiveCN111729129ADesign scienceSimple and fast operationAdditive manufacturing apparatusTissue regenerationMaterials sciencePolymerization
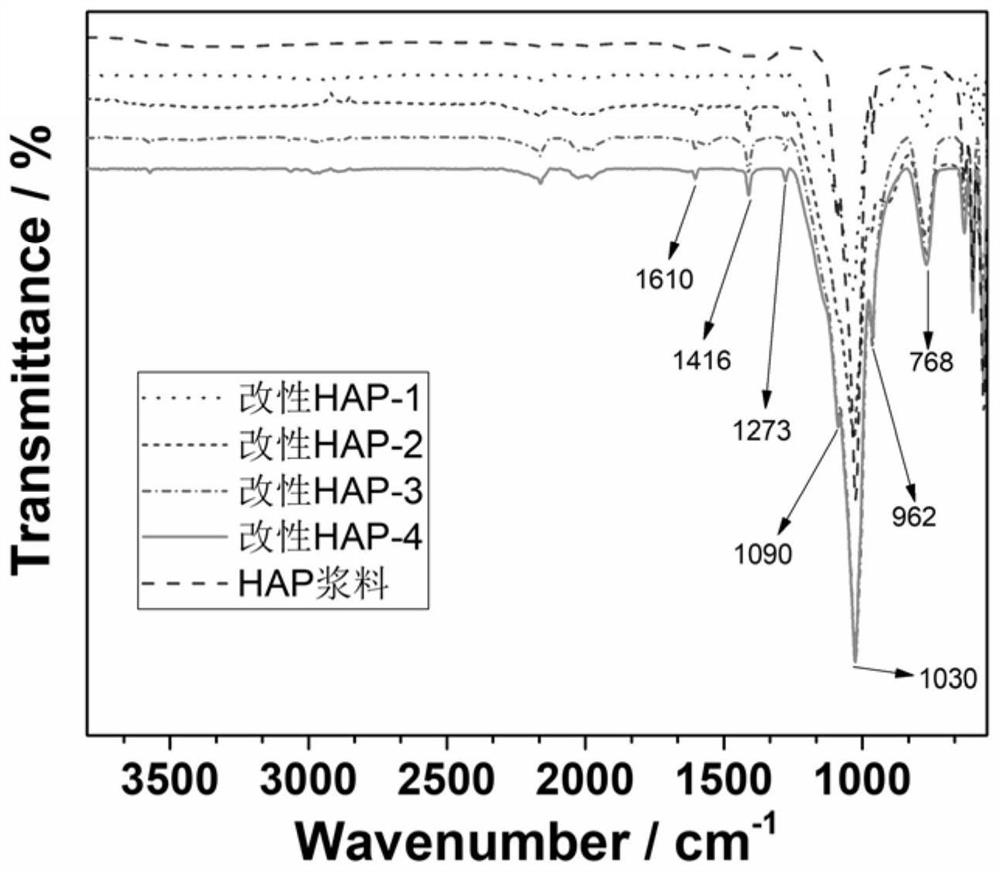
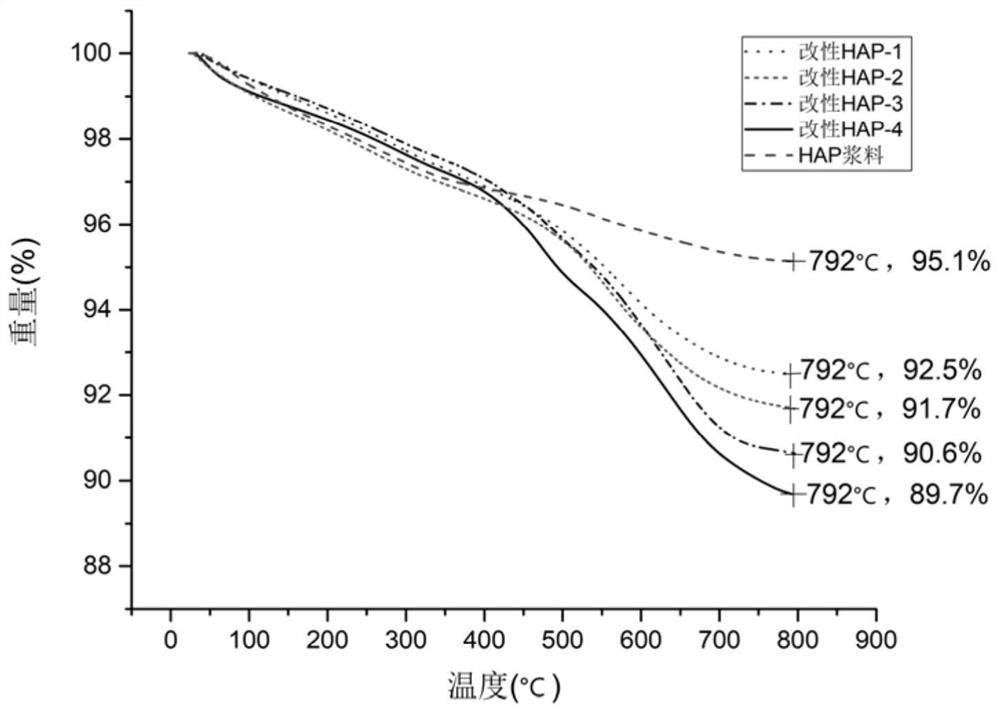
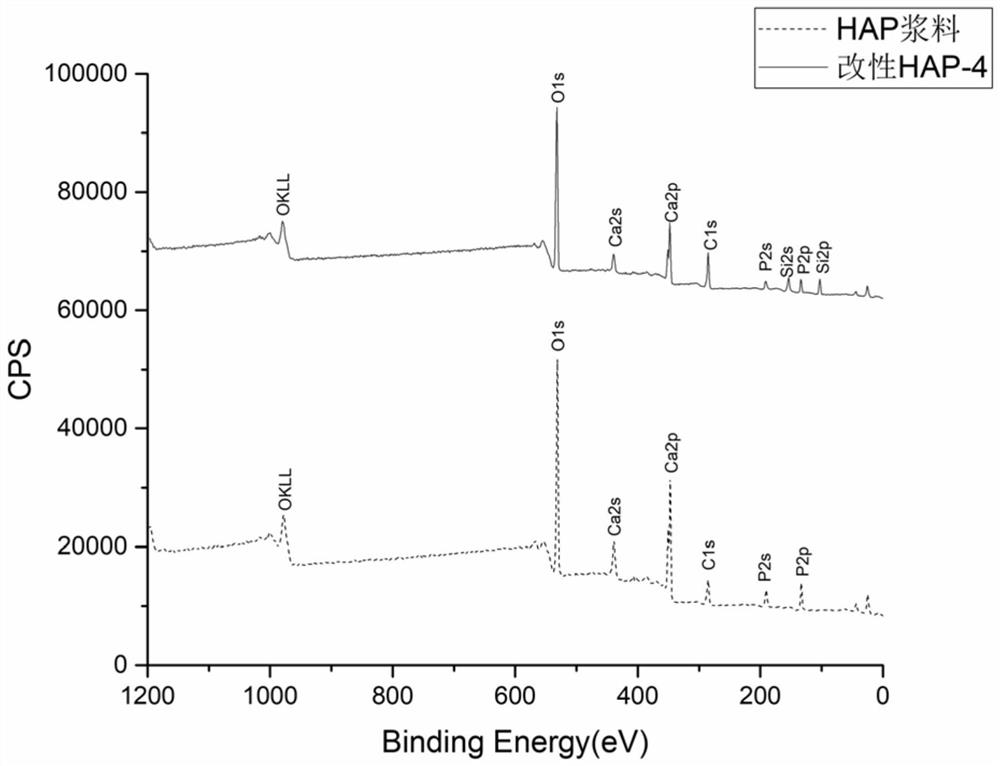
The invention belongs to the technical field of biomaterials and particularly relates to refined manufacturing of a photo-control-hybridized cross-linked degradable support and bone tissue engineeringapplication of the photo-control-hybridized cross-linked degradable support. A method for preparing the photo-control-hybridized cross-linked degradable support comprises the steps: 1) preparing modified hydroxyapatite; 2) preparing a bonder GelMA; and 3) blending a blue-light initiator and a thickener to prepare slurry, subjecting double bonds in the GelMA and double bonds of silane-coupler-modified grafted hydroxyapatite to an interaction, i.e., a formula shown in the description in the presence of free radicals through 3D printing technique forming under the condition of illumination of specific wavelength, then, initiating monomer polymerization, and carrying out bonding, thereby forming the solid-phase hybridized degradable support. The invention relates to application of the osteogenesis-remediation-promoting photo-crosslinked composite 3D-printed hybridized degradable support as a drug carrier or / and a bioscaffold material. The support is scientific in design and simple and convenient in operation and can be applied to carrier support materials of bone-defected regenerated remediation or all classes of bioactive substances due to refined controllable processing characteristics and biodegradable characteristics.
Owner:SICHUAN UNIV
Ultra-low temperature preservation solution for animal cells and preserving method
InactiveCN101037667AMaintain biological activityMaintain biological functionDead animal preservationTissue cultureBiotechnologyImproved survival
The invention discloses a ultra-low temperature conserving solution of animal cell and conserving method thereof for conserving cell especially fresh separated primary animal cell, e.g. liver cell. Culturing fresh separated animal cell at 37 DEG C 0-24 h, changing culture medium as ultra-low temperature conserving solution at 0-4 DEG C then conserving 0-48 months at -80--196 DEG C. After conserving finished, recoverying culturing in a fresh cell culture medium at 37 DEG C, finally culturing in a normal animal cell culture medium. Adding Chinese medicine effective constituent and other chemical protective agent in conserving process and before and after culture medium to improve survival rate of animal cell after conserving at ultra-low temperature and function of cell. The ultra-low temperature conserving technique of separated animal cell is used for conveying and conserving biotype artifical organs in consist of biology artifical liver and other organ cell, also suit for application of animal cell in other medicine field.
Owner:ZHEJIANG UNIV
Culture method of mouse lung organs and special culture solution thereof
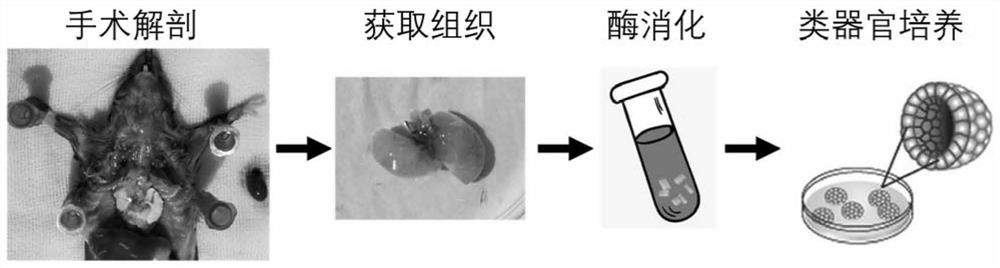
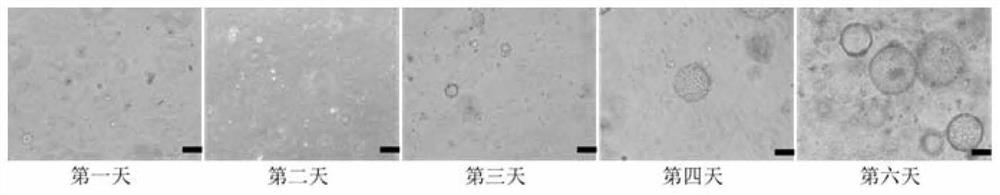
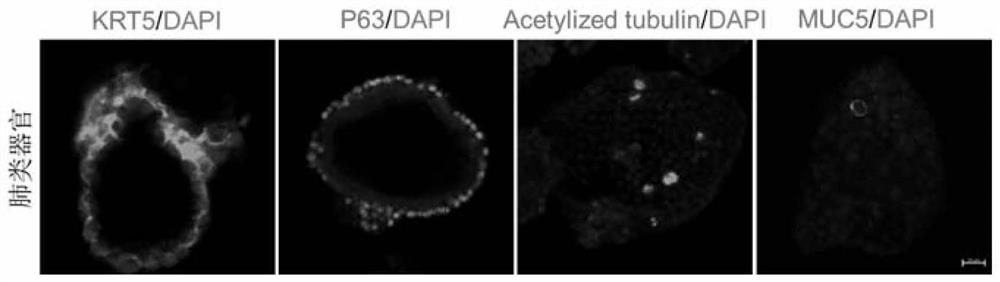
PendingCN113151152AEffective maintenance of interactionsMaintain heterogeneityCulture processArtificial cell constructsStainingImmunofluorescent stain
The invention discloses a culture method of mouse lung organs and a special culture solution thereof. The method comprises the following steps: acquiring fresh mouse lung tissue cells, digesting into single cells by collagenase, culturing mouse lung tissue organoid under an in-vitro three-dimensional culture condition, and performing immunofluorescent staining on the lung organoid. The culture solution is prepared from ADF < + + + + >, an RPSO1 conditional culture medium, a Noggin conditional culture medium, a B27 additive, N-acetyl-L-cysteine, nicotinamide, Y-27632, A83-01, SB202190, a recombinant human fibroblast growth factor-7 and a recombinant human fibroblast growth factor-10. Experiments show that the lung organ established by the method disclosed by the invention can effectively maintain tissue cell specificity, stem cell characteristics and biological functions, and meets the requirements of scientific research.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Culture and cryopreservation method of amniotic mesenchymal stem cells
ActiveCN111139221AHigh yieldImprove survival rateCell dissociation methodsCulture processStem Cell IsolationNeutral protease
The invention relates to a culture and cryopreservation method of amniotic mesenchymal stem cells. The culture method comprises amniotic tissue separation, amniotic mesenchymal stem cell separation, P0 generation amniotic mesenchymal stem cell culture and amplification culture. According to the culture method of the amniotic mesenchymal stem cells, at a separating stage of the amniotic mesenchymalstem cells, a special mixed enzyme digestive juice system (the final concentrations of components in the digestive juice are as follows: 1.5-2U / mL of neutral protease, 0.5mg / mL of deoxyribonuclease Iand 1mg / mL of collagenase IV) is adopted for digestion, so that the amniotic mesenchymal stem cells can be more effectively separated from amniotic tissues, and then the yield of P0 generation cellsis obviously improved. Compared with conventional two-step digestion, the culture method provided by the invention has the advantages that a one-step digestion method is adopted, operation is more convenient, and the cultured amniotic mesenchymal stem cells have good activity, high yield, high purity and good repeatability.
Owner:赛瑞诺(北京)生物科技有限公司
Stem cell cryopreservation liquid as well as preparation method and application thereof
ActiveCN114041455ANo damageReduced Possibility of ContaminationDead animal preservationBiotechnologyCell phenotype
The invention discloses a stem cell cryopreservation solution as well as a preparation method and application thereof. The cryopreservation liquid is prepared from the following components in percentage by volume: 20 to 40 percent of a basic culture medium, 10 to 20 percent of glycerol, 30 to 60 percent of human serum albumin, 2 to 6 percent of trehalose, 0.5 to 2 percent of glycine or taurine and 6 to 10.5 percent of inositol galactoside or glycoside. According to the cryopreservation liquid, the serum-free cryopreservation liquid is adopted for cryopreservation of the stem cells, the cells are free of damage, the possibility of animal pathogen pollution can be reduced, the inositol galactoside or the glycoside serves as the stabilizer, the cell phenotype can be effectively protected, the activity of the stem cells under the cryopreservation condition can be remarkably improved, and therefore, the physiological function and the biological function of the recovered cells are maintained.
Owner:东莞再立健生物科技有限公司
Human umbilical cord mesenchymal stem cell injection cryopreservation liquid with high multiplication capacity
PendingCN112841175ASo as not to damageGood freezing effectDead animal preservationSodium Chloride InjectionMesenchymal stem cell
The invention discloses a human umbilical cord mesenchymal stem cell injection cryopreservation solution with high multiplication capacity, which is prepared from the following raw materials in parts by volume: 1 to 5 parts of DMSO, 45 to 55 parts of compound electrolyte injection, 5 to 15 parts of dextran 40 glucose injection, 1 to 10 parts of sodium chloride injection, 1 to 10 parts of glucose injection, 20 to 40 parts of human serum albumin, and 3 to 6 parts of a serum substitute. The invention further discloses a preparation and cryopreservation method of the human umbilical cord mesenchymal stem cells, the content of DMOS is reduced, so that the clinical use risk is reduced, the human umbilical cord mesenchymal stem cells still keep a good cryopreservation effect, the human umbilical cord mesenchymal stem cells have high survival rate after cryopreservation and resuscitation, and meanwhile, the high value-added ability is kept. The proliferation capacity of unfrozen and resuscitated cells after 6 months of cryopreservation in the refrigerating fluid is equivalent to the proliferation capacity of fresh cells, and is far higher than the proliferation capacity of unfrozen and resuscitated cells after 6 months of cryopreservation in traditional refrigerating fluid.
Owner:朱灏
Low-temperature preservation and transportation culture medium
ActiveCN111100838ALong-term organizational vitalityIncrease energy metabolismEpidermal cells/skin cellsCulture processSucroseVitamin C
The invention provides a low-temperature preservation and transportation culture medium. The low-temperature preservation and transportation culture medium comprises the following components: a melanin skin model culture solution, a buffer system, agarose, sucrose, glycine, adenosine, alpha-tocopherol, vitamin C, sphingosine-1-phosphoric acid, Y-27632 and heparin, wherein the melanin skin model culture solution comprises a basic culture solution, hydrocortisone, insulin, growth factors, epinephrine, 12-myristic acid-13-phorbol acetate, stem cell factors, glutamine, calcium chloride and antibiotics.
Owner:GUANGDONG BOXI BIO TECH CO LTD
Preparation method of human umbilical cord mesenchymal stem cell injection cryopreservation liquid
ActiveCN112889811ASmall side effectsGuaranteed functionDead animal preservationSodium Chloride InjectionBiocompatibility
The invention relates to the technical field of cryopreservation liquid preparation, in particular to a preparation method of a human umbilical cord mesenchymal stem cell injection cryopreservation liquid. The preparation method of the human umbilical cord mesenchymal stem cell injection cryopreservation liquid at least comprises the following steps: adding a glucose injection, a sodium chloride injection, dextran and a compound electrolyte injection into dimethyl sulfoxide, and uniformly mixing; and then adding the human serum albumin, and uniformly mixing to obtain the cryoprotectant. The preparation method of the human umbilical cord mesenchymal stem cell injection cryopreservation liquid is optimized, so that the prepared cryopreservation liquid has high stability, no floccules or precipitates are generated in the preparation process and after the preparation process is completed, the functional indexes and biological functions of human umbilical cord mesenchymal stem cells are maintained, and the safety and the biocompatibility of the human umbilical cord mesenchymal stem cell back transfusion to a human body are improved.
Owner:朱灏
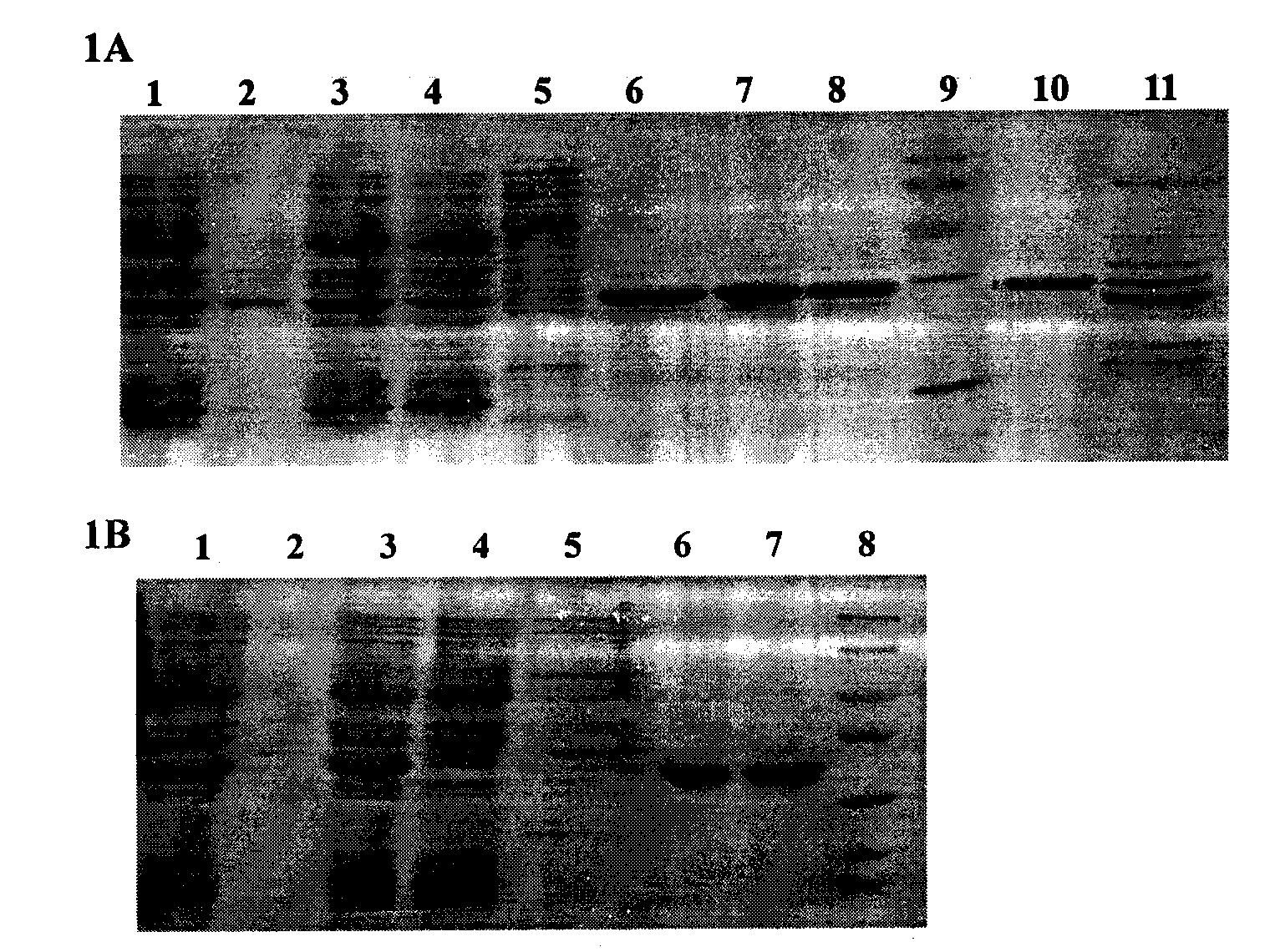
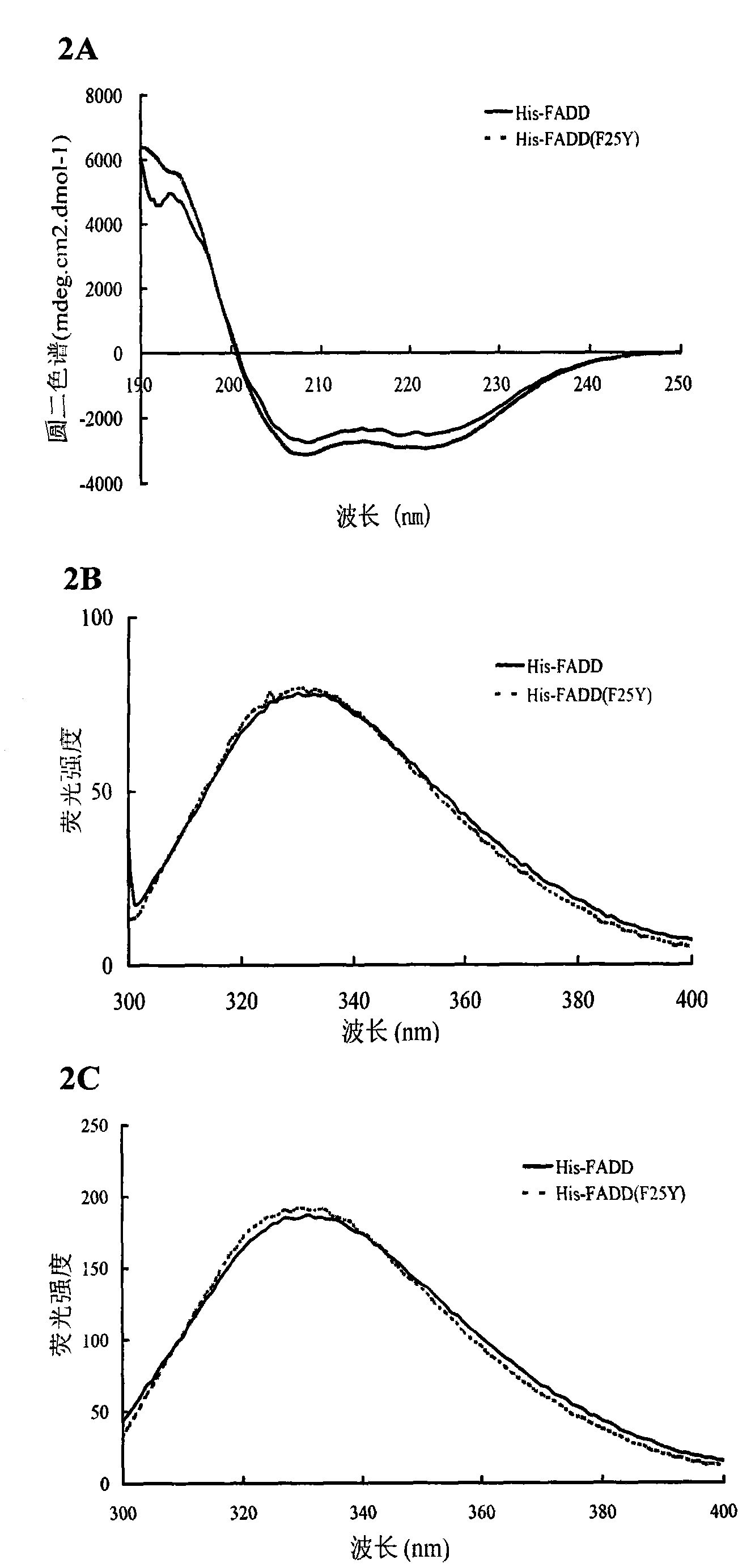
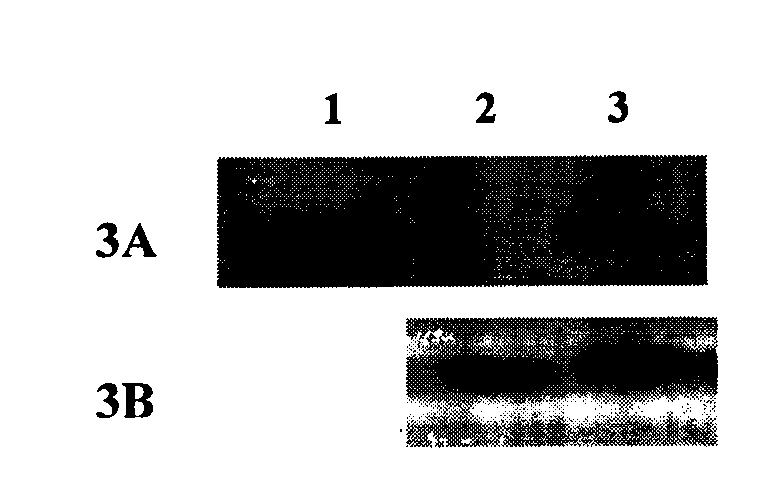
Preparation method of recombinant Fas-associated death domain protein and application thereof
ActiveCN101629181BEfficient expressionEasy to separatePeptide/protein ingredientsSkeletal disorderAbnormal tissue growthDisease
The invention belongs to the technical field of biology, in particular to a preparation method of full-length Fas-associated death domain (FADD) protein, which utilizes a recombinant DNA technique to express and prepare the full-length FADD protein which has good stability, high yield, the same high-level structure as a wild FADD and activity for inducing cell apoptosis, and the preparation method has more convenient and faster separation and purification process and high yield. The full-length FADD protein can be applied to prepare a medicament for inducing the cell apoptosis and treat diseases such as tumors, rheumatoid arthritis, and the like. The invention also provides an expression method for expressing the full-length FADD protein in a prokaryotic expression system, which can effectively improve the yield of the full-length FADD protein.
Owner:NANJING UNIV
Method and medium for amplifying bone-marrow mesenchymal stem cells and application
PendingCN110172445AImprove proliferative abilityMaintain biological functionSkeletal/connective tissue cellsCell culture active agentsChronic myocardial infarctionCardiac failures
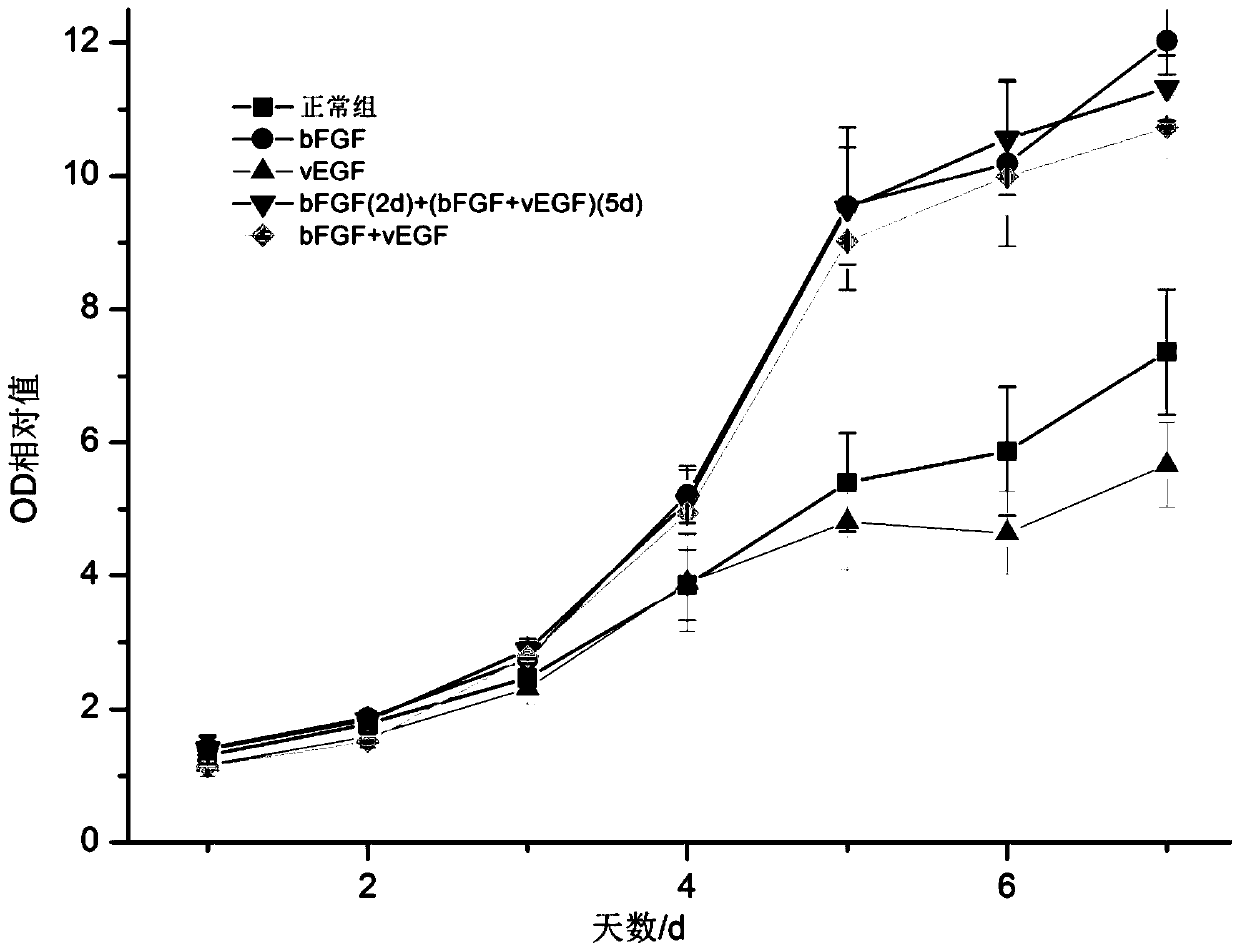
The invention relates to the technical field of bioengineering, and particularly relates to a method and a medium for amplifying bone-marrow mesenchymal stem cells and application. The method for amplifying bone-marrow mesenchymal stem cells comprises the steps of treating the bone-marrow mesenchymal stem cells with bFGF and VEGF to amplify the bone-marrow mesenchymal stem cells. The bFGF and VEGFare added to the medium. The bone-marrow mesenchymal stem cells cultured by the method provided by the invention can increase the proliferation ability of the cells, and maintain the biological characteristics and functions of the cells, and also can increase the expression rate of the cell surface molecule CD105, thereby being useful for transplant treatment of acute myocardial infarction, chronic myocardial infarction, and ischemic heart disease caused by cardiac failure.
Owner:SOUTH CHINA INSTITUDE OF BIOMEDICINE +1
Invitro tissue cultivation method of hepatocyte of mammal
InactiveCN101775365AImprove adsorption capacityGood for mutual contactArtificial cell constructsVertebrate cellsMammalCulture mediums
The invention relates to an invitro tissue cultivation method of hepatocyte of a mammal, adopting a hepatocyte culture medium with a three-dimensional porous structure of static tissue. The medium has a diameter of 1-2mm and a thickness of 3-4mm; the medium is provided with holes differing in size, wherein the diameter of the hole ranges from 80mum-500mum, and holes are communicated with each other. The method ensures that the hepatocyte of human or animal is rapidly multiplied during culture in vitro and keeps the cell biology and function of the hepatocyte cell.
Owner:苑国忠
A kind of magnesium-based metal material conversion film and preparation method thereof
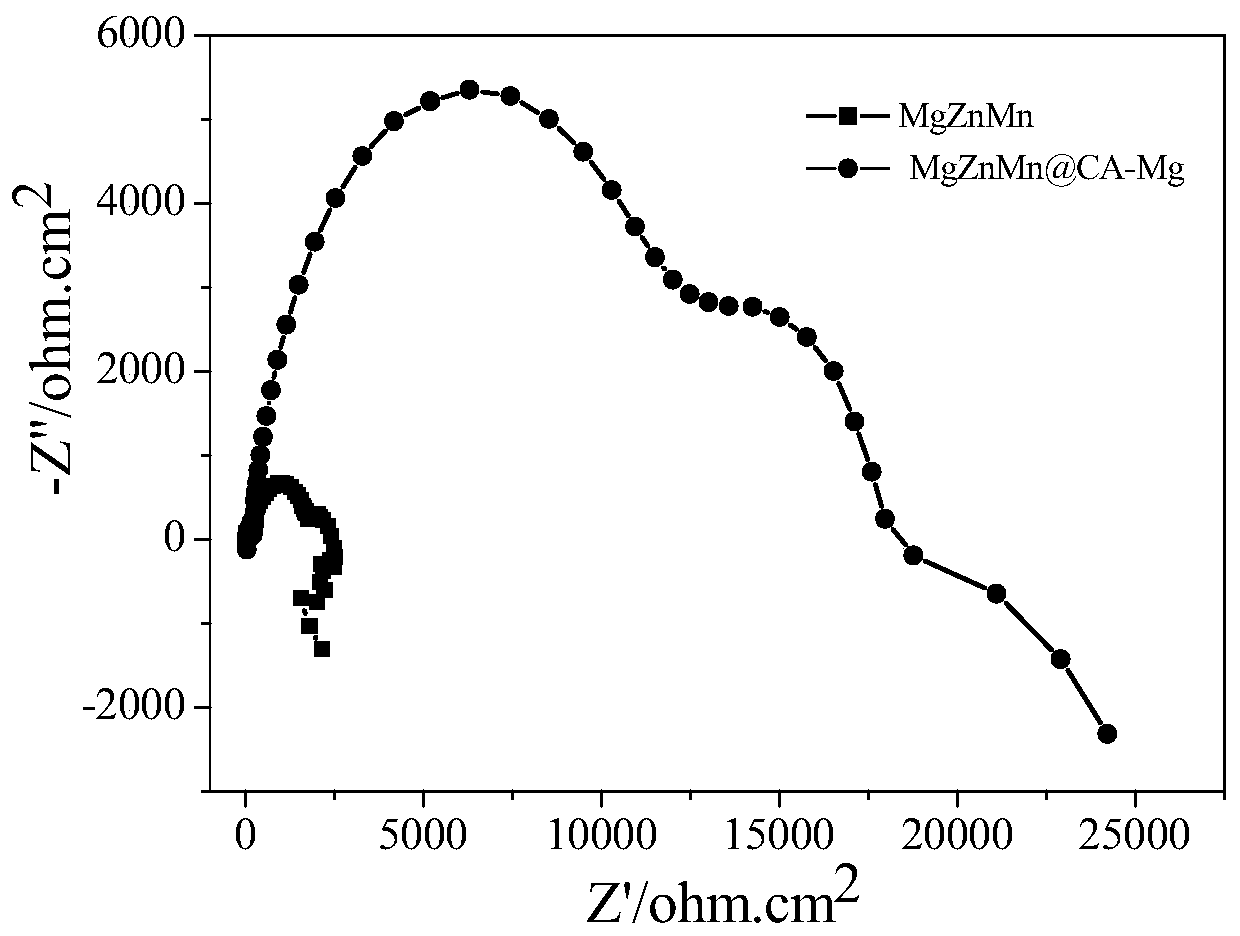
ActiveCN109055926BReduce the degradation rateReduce investmentMetallic material coating processesCardiovascular stentMetallic materials
Owner:SICHUAN UNIV
In-vitro separation and culture method of sustentacular cell of Mongolian horse
PendingCN111088215AGood technical effectAvoid stickingCell dissociation methodsArtificial cell constructsEnzyme digestionSustentacular cell
The invention provides an in-vitro separation and culture method of a sustentacular cell of Mongolian horse. The method comprises the steps of tissue dissociation and washing, tissue digestion, cell inoculation, cell culture and maintenance and the like. According to the method, the whole testicular tissue of a large-sized mammal Mongolian horse is subjected to tissue separation and culture so asto acquire the sustentacular cell. The method is simple in procedure and low in cost, can be used for effectively removing aggregate tissues generated in an enzyme digesting process, so that the activity and biological functions of the cell can be maintained; and impure cells generated in the separation and culture process can be effectively removed, the sustentacular cell separation efficiency can be improved, and foundation is provided to subsequent germ cell researches.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
A kind of culture and cryopreservation method of amniotic mesenchymal stem cells
ActiveCN111139221BHigh yieldImprove survival rateCell dissociation methodsCulture processStem Cell IsolationNeutral protease
The invention relates to a method for culturing and freezing amniotic mesenchymal stem cells. The culturing method includes amniotic membrane tissue separation, amniotic mesenchymal stem cell separation, P0 generation amniotic mesenchymal stem cell culture and expansion culture. The culture method of amniotic mesenchymal stem cells of the present invention, in the separation stage of amniotic mesenchymal stem cells, adopts a special mixed enzyme digestive solution system (final concentration of each component in the digestive solution: 1.5-2U / mL neutral protease, 0.5mg / mL deoxyribonuclease I and 1mg / mL collagenase IV) for digestion, can more effectively separate amniotic mesenchymal stem cells from amniotic tissue, and then significantly improve the yield of P0 generation cells. The present invention adopts one-step digestion Compared with the traditional two-step digestion method, the operation is more convenient, and the cultured amniotic mesenchymal stem cells have good activity, high yield, high purity, and good repeatability.
Owner:赛瑞诺(北京)生物科技有限公司
T lymphocyte cryopreservation solution
The invention discloses a T lymphocyte cryopreservation solution, which is prepared from the following raw materials by volume: 1-5 parts of DMSO, 5-15 parts of dextran 40 glucose injection, 40-60 parts of compound electrolyte injection, 20-40 parts of human serum albumin and 5-15 parts of Dulbecco's medium or Dulbecco's modified medium. The invention also discloses a preparation method and a cryopreservation method thereof, the content of DMOS is reduced, so that the clinical use risk is reduced, the T lymphocyte still keeps a good cryopreservation effect, and the T lymphocyte has high survival rate and keeps a better form after cryopreservation and resuscitation. Besides, in the preparation process, the phenomenon that the human serum albumin generates protein precipitation when encountering DMSO is also solved, cells cryopreserved by the cryopreservation solution can be directly diluted and then applied clinically, components of the cryopreservation solution do not need to be centrifugally removed, and the cryopreservation solution can be used as an auxiliary material and is directly applied to clinical administration, so that the cryopreservation solution is more convenient to use.
Owner:朱灏
Methods and system for amplifying bone marrow mesenchymal stem cells, and computer readable medium
PendingCN110205289AImprove proliferative abilityProliferation effect is goodCulture processSkeletal/connective tissue cellsEndothelial differentiationProliferative capacity
The invention relates to methods and system for amplifying bone marrow mesenchymal stem cells, and a computer readable medium. The method includes performing induction culturing on bone marrow mesenchymal stem cells for 1-3 days under conditions that temperature is at 36-38 DEG C, the volume concentration of N2 is 91-93%, the volume concentration of air is 1%, the volume concentration of CO2 is 4.5-5.5% and the volume concentration of O2 is 1.5-2.5%; and performing continuous culturing on the induction-cultured bone marrow mesenchymal stem cells under the conditions that the temperature is at36-38 DEG C, the volume concentration of CO2 is 4.5-5.5% and the volume concentration of O2 is 15-25% so as to amplify the bone marrow mesenchymal stem cells. The method can rapidly amplify the bone marrow mesenchymal stem cells and obtain the bone marrow mesenchymal stem cells with the best proliferative capacity, biological activity and endothelial differentiation function.
Owner:SOUTH CHINA INSTITUDE OF BIOMEDICINE +1
Double-layer cell co-culture device with adjustable gap
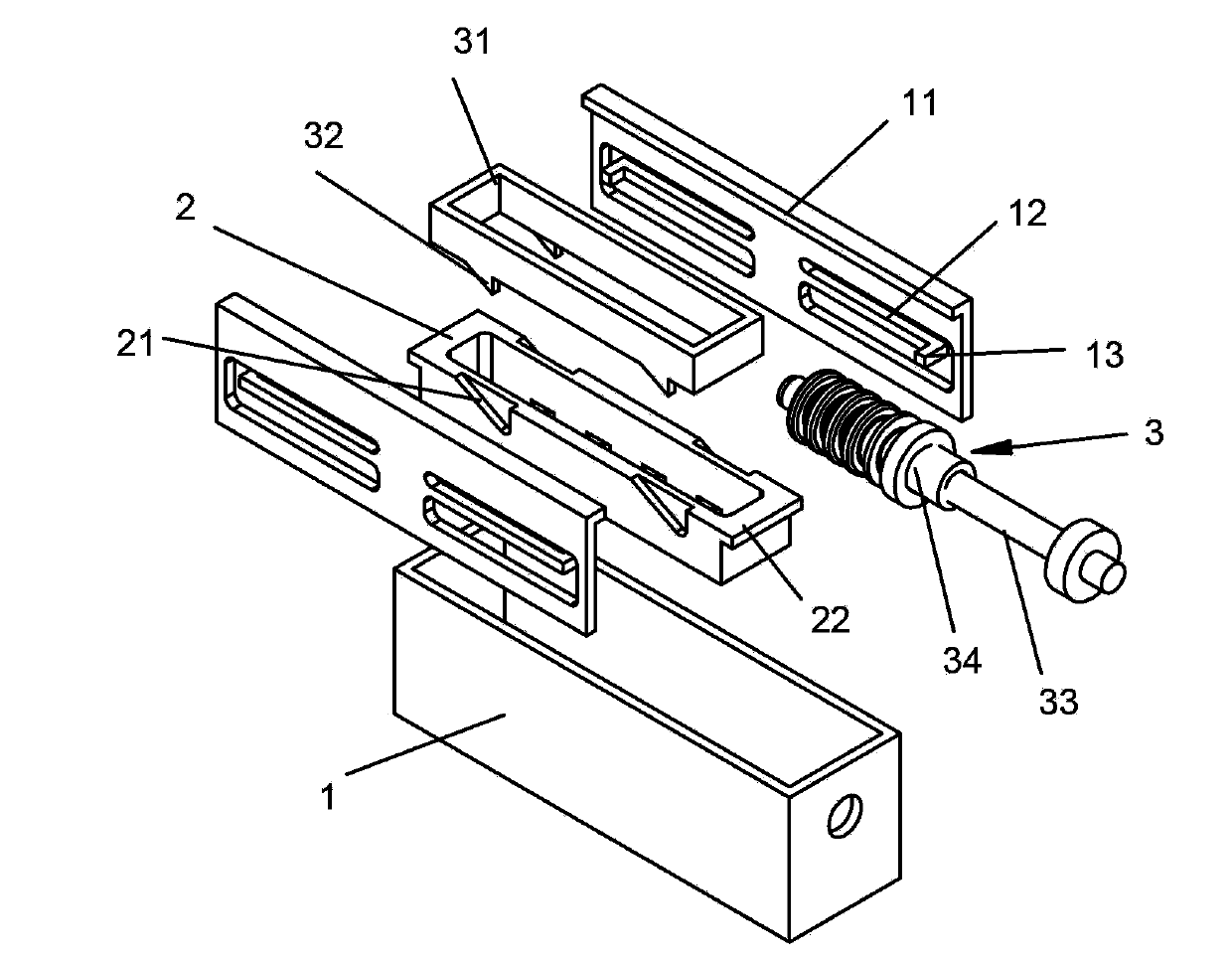
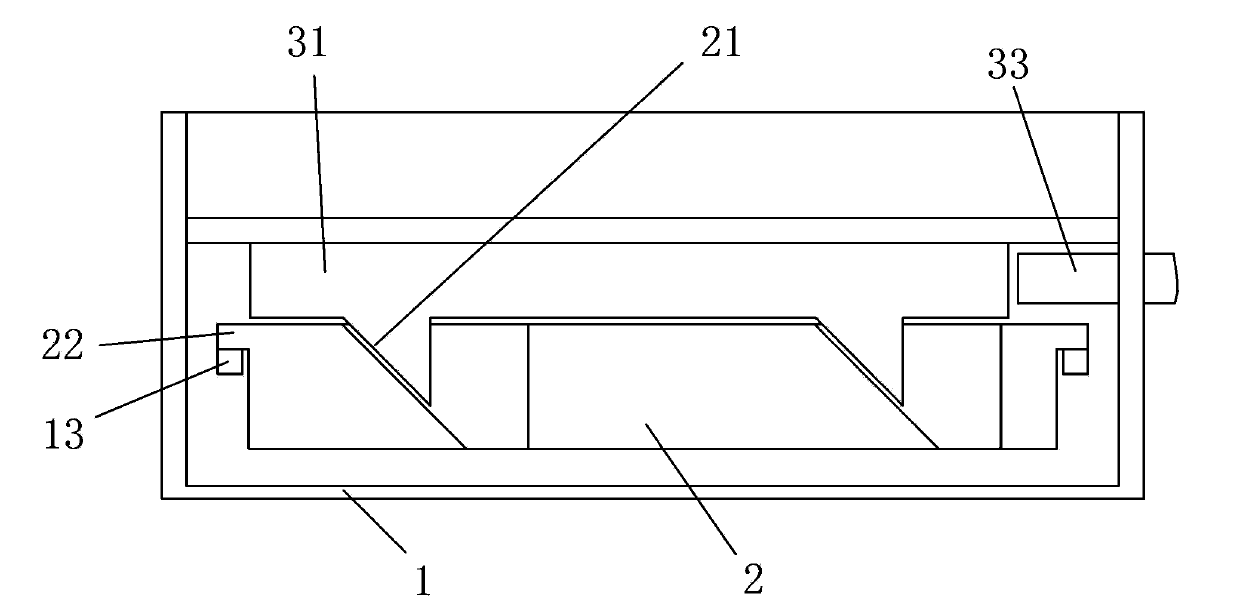
ActiveCN103194388BPromote proliferationMaintain biological functionTissue/virus culture apparatusCell growthCell biology
The invention discloses a double-layer cell co-culture device with an adjustable gap. The double-layer cell co-culture device comprises a cell culture box, a cell culture groove and a driving part, wherein the cell culture box is used for culturing a first cell; the cell culture groove is used for culturing a second cell, and is arranged in the cell culture box in a removable manner; and the driving part drives the cell culture groove to move to the bottom of the cell culture box in the cell culture box, and can adjust the gap between the bottom of the cell culture box and the bottom of the cell culture groove to the predetermined distance. According to the double-layer cell co-culture device provided by the invention, the driving part drives the cell culture groove to move to the bottom of the cell culture box, and thus, the distance between the double layers of cells is adjusted in an on-line manner, the gap can adjust the growth and the function of the cells, so that the adjustable growing environment is provided for cell co-culture, and the feasible device is provided for exploring a cell co-culture technology.
Owner:INST OF MECHANICS - CHINESE ACAD OF SCI
A kind of solid culture medium and preservation method thereof for skin model cryopreservation
ActiveCN104222068BMaintain biological activityMaintain biological functionDead animal preservationCulture fluidCryopreservation
The invention discloses a low-temperature preserved solid culture medium used for a tissue engineering skin model and a preservation method of the low-temperature preserved solid culture medium. The low-temperature preserved solid culture medium is prepared by the following steps: adding a low-temperature protection agent into a common culture medium for epidermis cells to prepare a basic culture solution; and mixing the basic culture solution with agarose gel and condensing to form the solid culture medium suitable for the skin model. The tissue engineering skin model is embedded into the culture medium, is preserved at 4-8 DEG C for 24-72 hours, and then is resuscitated by a common epidermis cell culture solution. The solid culture medium can be used for reducing tissue activity reduction and structure damages of cells and skin tissues, caused by a low-temperature preservation condition, to the greatest extent.
Owner:GUANGDONG BOXI BIO TECH CO LTD
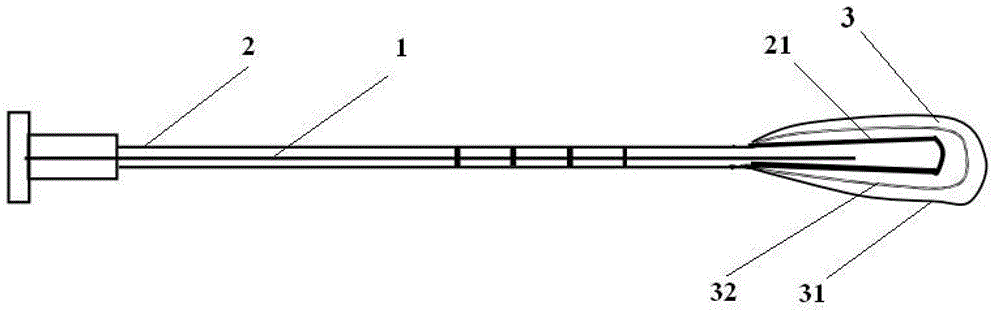
Degradable and foldable biological amnion composite repair stent
ActiveCN109394398AImprove fitPromote repairStentsAdditive manufacturing apparatusInsertion stentLacrimal duct
The invention discloses a degradable and foldable biological amnion composite repair stent. The degradable and foldable biological amnion composite repair stent comprises a tube-shaped body with an axially extending through hole, an elastic balloon is arranged at the front end of the tube-shaped body, a one-way valve is connected to the tail end of the tube-shaped body, the one-way valve closes the through hole at the position, a foldable net-pipe-shaped polylactic acid bracket covers the outer surface of the elastic balloon, a biological amnion covers the outer surface of the foldable net-pipe-shaped polylactic acid bracket, a plurality of tiny holes are formed in net filaments of the foldable net-pipe-shaped polylactic acid bracket, and biological amnion powder fills the plurality of tiny holes; under the initial state, the elastic balloon, the foldable net-pipe-shaped polylactic acid bracket and the biological amnion are compressed to in the compact state; and under the use state, after the stent is implanted into the body and swelling occurs due to pressurizing, the tube-shaped or waterdrop-similar-shaped state can be formed complying with the lacrimal duct / uterine cavity, or the other spatial states adaptive to body cavity also can be formed.
Owner:JIANGXI RUIJI BIOTECH CO LTD
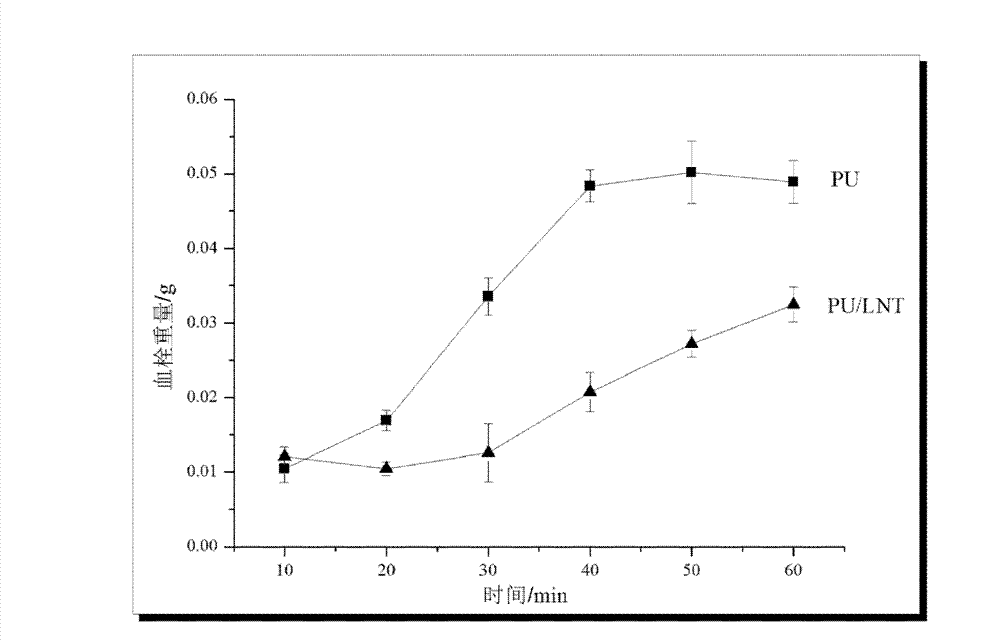
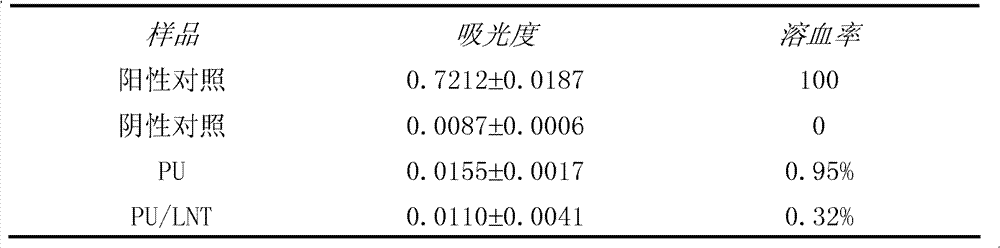
Polyurethane material subjected to photo-induced graft surface modification by fungi polysaccharide and preparation method thereof
InactiveCN102181068BGood biocompatibilityImprove surface biological propertiesArtificial organAntibacterial activity
The invention relates to a polyurethane material subjected to photo-induced graft surface modification by fungi polysaccharide and a preparation method thereof. The polyurethane material subjected to photo-induced graft surface modification by the fungi polysaccharide is characterized by consisting of a base material and a modification layer; the base material consists of a common commercial polyurethane material; and the modification layer is formed by performing photo-induced graft surface modification on the surface of the base material by using fungi polysaccharide, namely lentinan. The surface of the polyurethane material subjected to photo-induced graft surface modification by the fungi polysaccharide has high antibacterial activity, and blood compatibility, so that as the artificial transplant material implanted into a human body, and the biocompatible medical material of an artificial organ and device, the polyurethane material has a wide application range, and the preparationmethod of the material is simple, high in reaction speed, and low in cost and is easy to control.
Owner:WUHAN UNIV OF TECH
Degradable compound active amnion material, preparation method and application thereof and compound active amnion uterine cavity repair stent
ActiveCN103349798BStructure remains intactMaintain biological functionSurgeryMedical devicesSacculeFreeze-drying
The invention provides a compound active amnion material, a preparation method and application thereof and a compound active amnion uterine cavity repair stent. The preparation method comprises the following steps: (1) providing a collagen sponge prepare liquid or a compound collagen sponge prepare liquid; (2) providing an amnion with both an epithelial layer and a basement membrane; (3) pouring and coating the prepared prepare liquid on the basement membrane of the amnion to enable the basement membrane and the prepare liquid to be subject to crosslinking, and then preparing the compound active amnion material through freeze drying. The compound active amnion material not only keeps the complete structure and the due biological function of the amnion but also endow the amnion with a certain rigidity, so that the compound active amnion material has stronger maneuverability in application after TCRA (Transcervical Resection Of Adhesions) operation; the compound active amnion uterine cavity repair stent integrates the compound active amnion material and a saccule at the front end of a pipe body, the saccule expands under the action of an external force to push out the compound active amnion material, and then the compound active amnion material is successfully attached to the inner wall of the uterine cavity; the stent is convenient to take out and prevents secondary damage.
Owner:JIANGXI RUIJI BIOTECH CO LTD
Ultra-low temperature preservation solution for animal cells and preserving method
InactiveCN100519742CMaintain biological activityMaintain biological functionDead animal preservationTissue cultureBiotechnologyImproved survival
The invention discloses a ultra-low temperature conserving solution of animal cell and conserving method thereof for conserving cell especially fresh separated primary animal cell, e.g. liver cell. Culturing fresh separated animal cell at 37 DEG C 0-24 h, changing culture medium as ultra-low temperature conserving solution at 0-4 DEG C then conserving 0-48 months at -80--196 DEG C. After conserving finished, recoverying culturing in a fresh cell culture medium at 37 DEG C, finally culturing in a normal animal cell culture medium. Adding Chinese medicine effective constituent and other chemical protective agent in conserving process and before and after culture medium to improve survival rate of animal cell after conserving at ultra-low temperature and function of cell. The ultra-low temperature conserving technique of separated animal cell is used for conveying and conserving biotype artifical organs in consist of biology artifical liver and other organ cell, also suit for application of animal cell in other medicine field.
Owner:ZHEJIANG UNIV
Preparation method of recombinant Fas-associated death domain protein and application thereof
ActiveCN101629181AEfficient expressionEasy to separatePeptide/protein ingredientsMicroorganism based processesAbnormal tissue growthDisease
The invention belongs to the technical field of biology, in particular to a preparation method of full-length Fas-associated death domain (FADD) protein, which utilizes a recombinant DNA technique to express and prepare the full-length FADD protein which has good stability, high yield, the same high-level structure as a wild FADD and activity for inducing cell apoptosis, and the preparation method has more convenient and faster separation and purification process and high yield. The full-length FADD protein can be applied to prepare a medicament for inducing the cell apoptosis and treat diseases such as tumors, rheumatoid arthritis, and the like. The invention also provides an expression method for expressing the full-length FADD protein in a prokaryotic expression system, which can effectively improve the yield of the full-length FADD protein.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com