Preparation method of adsorbent for adsorbing and separating CO2
An adsorption separation and adsorbent technology, applied in the field of adsorbent preparation, can solve the problems of small adsorption capacity, poor cycle stability, and low adsorption selectivity, and achieve high adsorption capacity, high adsorption selectivity, high adsorption capacity and selectivity sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Measure 9.0ml DMF and 1.0ml acetic acid into a 25ml beaker, and then add 25.5mg terephthalic acid, 15.3mg 2-sulfonic acid terephthalic acid monosodium salt and 53mg anhydrous tetrachloride into the beaker. zirconium. After stirring the above mixture uniformly, it was transferred to a 20ml hydrothermal reactor, and then placed in an oven to crystallize at 120°C for 40 hours. Naturally cooled to room temperature, centrifuged and washed with DMF and diethyl ether 3 times, and then centrifuged to obtain solid powder. The solid powder prepared above is placed in a drying box and dried at 120°C for 24 hours to obtain UiO-66-SO 3 H sample. Using the above synthesis method, UiO-66-SO was synthesized in multiple batches 3 H samples are available.
[0021] Modified sulfonated metal organic framework material UiO-66-SO after ammonia water treatment 3 H. Weigh 1.5g UiO-66-SO 3 The H solid powder was added to a round bottom flask with a volume of 100ml, and then 90.0ml of ammonia wa...
Embodiment 2
[0023] UiO-66-SO 3 The preparation method and process of H are exactly the same as those described in Example 1.
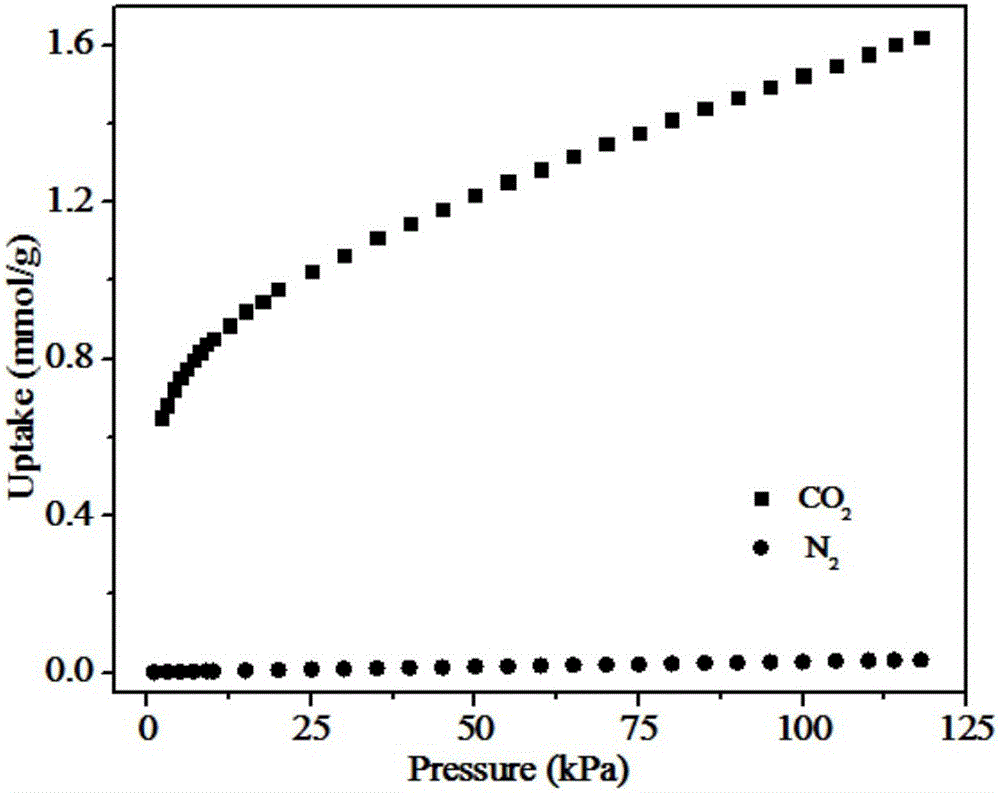
[0024] Modified sulfonated metal organic framework material UiO-66-SO after ammonia water treatment 3 H. Weigh 1.5g UiO-66-SO 3 The H solid powder was added to a round bottom flask with a volume of 100ml, and then 60.0ml of ammonia water with a concentration of 2.0M (ie ammonia water and UiO-66-SO 3 The mass ratio of H is 40:1); the mixture is placed in a water bath at a temperature of 60°C for 8 hours, cooled in an ice-water mixture at 0°C for 10 minutes, washed three times with deionized water and ethanol by centrifugation, and the white powder obtained is transferred to Dry in a drying oven at 100°C for 10 hours to obtain an adsorbent, which is marked as adsorbent A2. Measured by ASAP2020 physical adsorption instrument, the BET specific surface area and pore volume of adsorbent A2 are respectively 439m 2 / g and 0.265cm 3 / g, and at 25℃ and 0.15bar, it has 2 And N ...
Embodiment 3
[0026] UiO-66-SO 3 The preparation method and process of H are exactly the same as those described in Example 1.
[0027] Modified sulfonated metal organic framework material UiO-66-SO after ammonia water treatment 3 H. Weigh 1.5g UiO-66-SO 3 H solid powder was added to a round bottom flask with a volume of 100ml, and then 45.0ml of ammonia water with a concentration of 3.0M (ie ammonia water and UiO-66-SO 3 The mass ratio of H is 30:1); the mixture is placed in a water bath at a temperature of 50°C for 6 hours, cooled in an ice-water mixture at 0°C for 10 minutes, washed with deionized water and methanol by centrifugation three times, and the white powder obtained is transferred to Dry it in a drying box at 90°C for 15 hours to obtain an adsorbent, which is marked as adsorbent A3. Measured by ASAP2020 physical adsorption instrument, the BET specific surface area and pore volume of adsorbent A3 are respectively 423m 2 / g and 0.262cm 3 / g, and at 25℃ and 0.15bar, the adsorbent A3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore volume | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com