A catalyst for the direct preparation of aromatic compounds from synthesis gas and its preparation and application
A technology of aromatic compounds and catalysts, applied in physical/chemical process catalysts, molecular sieve catalysts, chemical/physical processes, etc., can solve problems such as escape of intermediate products, inability to activate, react, and uneven concentration distribution of two active sites. Achieve the effects of high aromatics selectivity, low methane selectivity, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
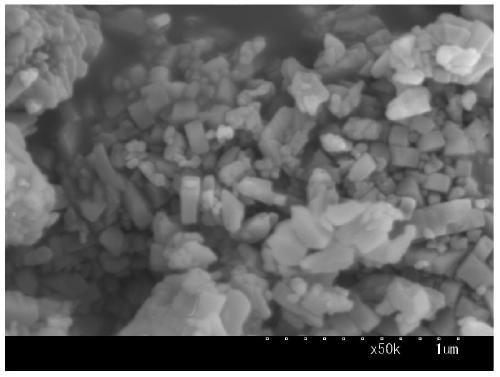
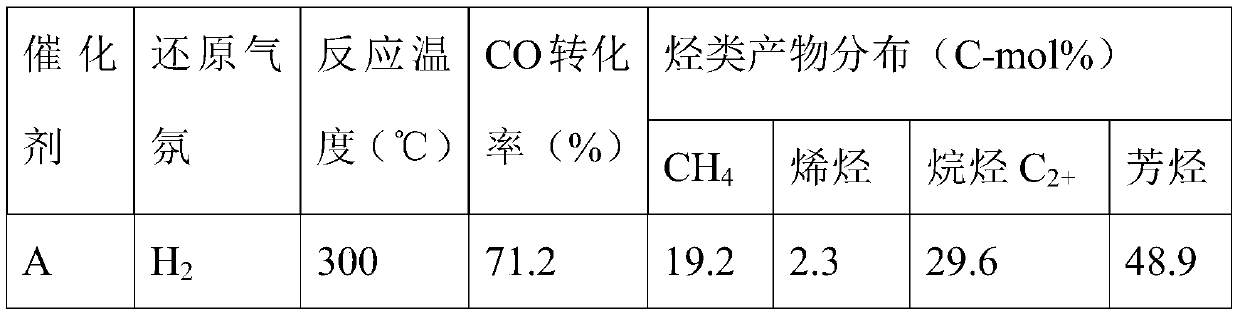
[0025] 11.84g ferric nitrate nonahydrate, 0.871g ammonium molybdate tetrahydrate and 0.644g potassium nitrate were dissolved in deionized water, and then impregnated onto 6.67g hydrogen ZSM-5 molecular sieve by equal volume co-impregnation. Rotary evaporation under vacuum to dryness, then drying in air at 120°C for 5h, and then calcining in air at 500°C for 5h, to obtain Catalyst A with iron oxide content of 23.4wt%, molybdenum oxide content of 7wt% and potassium oxide content of 3wt%. , the catalyst morphology is as figure 1 As shown in SEM, the average particle size of the catalyst is around 200nm.
Embodiment 2
[0027] Dissolve 9.39g of ferric nitrate nonahydrate, 1.14g of ammonium molybdate tetrahydrate and 1.06g of manganese nitrate in deionized water, and then impregnate 6.86g of hydrogen-type ZSM-5 molecular sieves in an excess volume co-impregnation method. Rotary evaporation under vacuum to dryness, and then drying in air at 120°C for 5h, followed by roasting in air at 500°C for 5h, to obtain iron oxide content of 18.6wt%, molybdenum oxide content of 8.6wt% and manganese oxide content of 4.2wt%. Catalyst B.
Embodiment 3
[0029] Dissolve 10.15g of ferric nitrate nonahydrate and 1.23g of potassium nitrate in deionized water, impregnate 6.72g of ZSM-5 molecular sieve by equal-volume impregnation method, rotary evaporate to dryness under vacuum, and then dry in air at 120°C for 5h. Next, the obtained sample was impregnated with a solution containing 0.90 g of ammonium molybdate tetrahydrate by an excess volume impregnation method, then evaporated to dryness under vacuum, then dried in air at 120°C for 5 hours, and then calcined in air at 500°C for 5 hours to obtain Catalyst C with an iron content of 20.1 wt%, a molybdenum oxide content of 6.8 wt% and a manganese oxide content of 5.7 wt%.
[0030] 2. The application of the invented catalyst in the conversion of synthesis gas to aromatics.
[0031] The prepared catalyst was shaped, crushed, and sieved under a pressure of 6.5 MPa to obtain a 40-60 mesh sample. Get 0.5g catalyst and place in the reactor of continuous flow, the catalyst before reactio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com