Preparation method of magnetoplumbite type CH4-CO2 reforming catalyst
A CH4-CO2, reforming catalyst technology, applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the problem of high energy consumption and the presence of many impurity phases , product impurity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
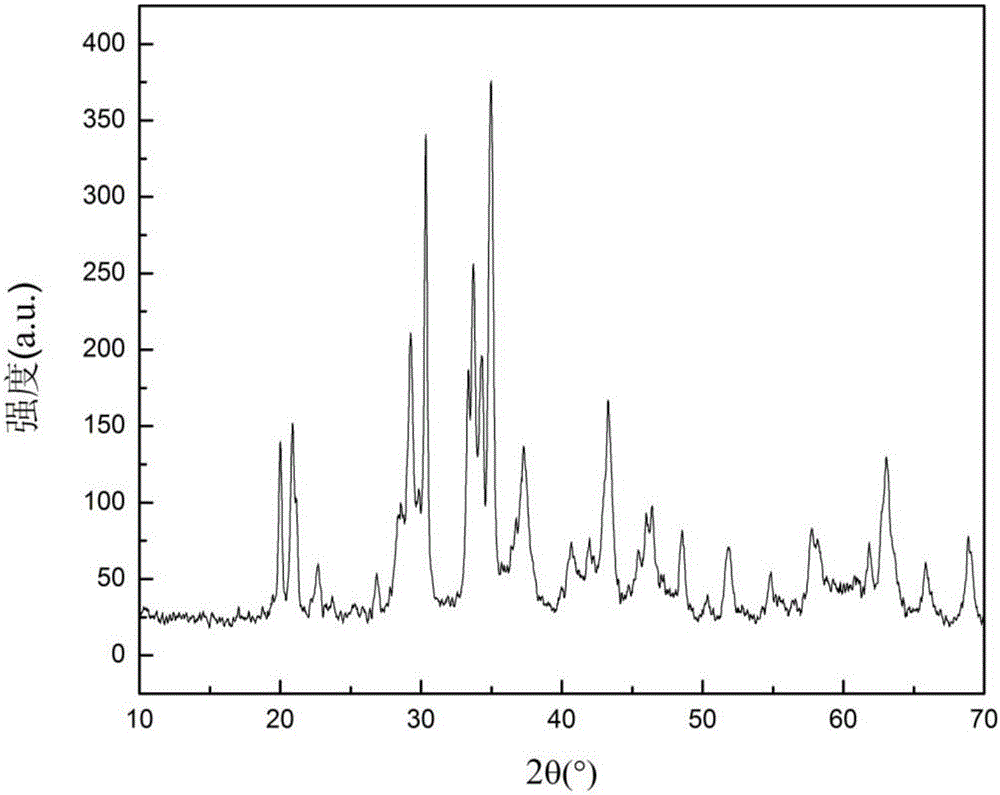
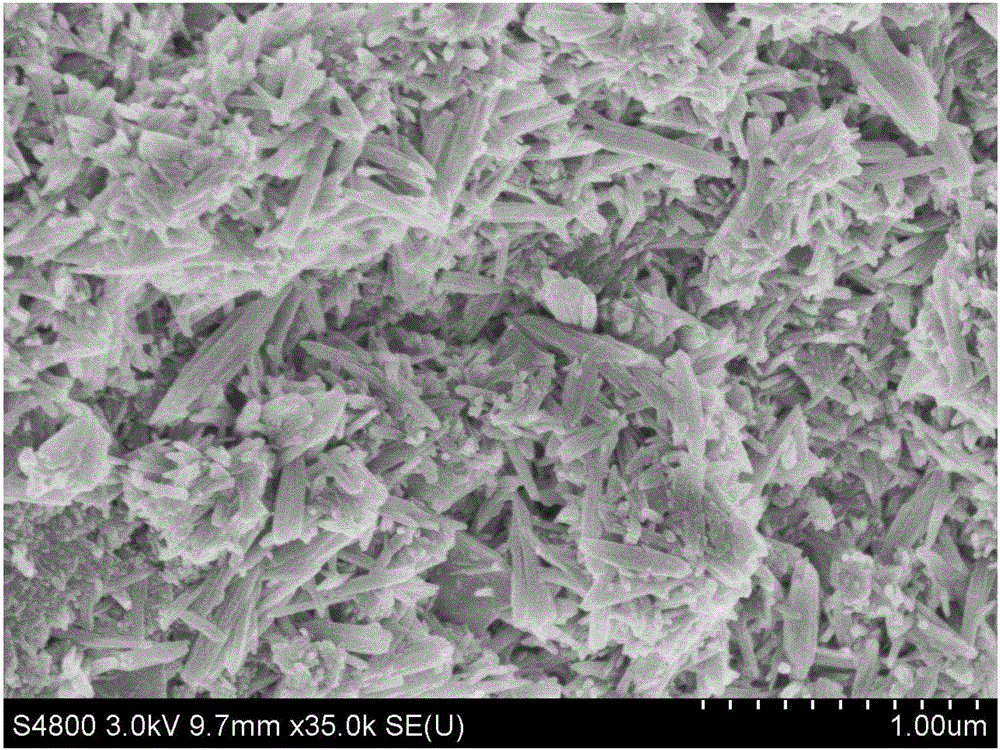
Image
Examples
Embodiment 1
[0037] (1) According to n(SrO):n(NiO):n(Al 2 o 3 )=1:1:6 molar ratio design magnetoplumbite structure oxide composition. First weigh a certain amount of sodium aluminate NaAlO 2 Chemical reagents, and use deionized water to prepare an aqueous solution with a concentration of 0.60 mol / L. Then weigh nickel nitrate Ni (NO 3 ) 2 ·6H 2 O and strontium nitrate Sr(NO 3 ) 2 Chemical reagents, dissolved in appropriate amount of deionized water.
[0038] (2) The sodium aluminate aqueous solution was placed in a digitally controlled temperature-adjusting ultrasonic instrument, and the nickel nitrate solution was first slowly added into it under the action of continuous stirring and ultrasonic waves at 50°C, and the strontium nitrate solution was continued to be slowly added after 10 minutes. Then the prepared mass concentration of 1% HNO 3 The solution was slowly added dropwise to the beaker, the pH value was adjusted to 9.5, and the ultrasonic stirring was continued for 20 min ...
Embodiment 2
[0042] (1) According to n(SrO):n(NiO):n(Al 2 o 3 )=1:1:6 molar ratio design magnetoplumbite structure oxide composition. First weigh a certain amount of sodium aluminate NaAlO 2 Chemical reagents, and use deionized water to prepare an aqueous solution with a concentration of 0.65mol / L. Then weigh nickel nitrate Ni (NO 3 ) 2 ·6H 2 O and strontium nitrate Sr(NO 3 ) 2 Chemical reagents, dissolved in appropriate amount of deionized water.
[0043] (2) The sodium aluminate aqueous solution was placed in a numerically controlled temperature-adjusting ultrasonic instrument, and the nickel nitrate solution was slowly added to it under the action of continuous stirring and ultrasonic waves at 45°C, and then the strontium nitrate solution was continued to be slowly added after 5 minutes. Then the prepared mass concentration of 1% HNO 3 The solution was slowly added dropwise into the beaker, the pH value was adjusted to 10, and the ultrasonic stirring was continued for 30 min to...
Embodiment 3
[0047] (1) According to n(SrO):n(NiO):n(Al 2 o 3 )=1:1:6 molar ratio design magnetoplumbite structure oxide composition. First weigh a certain amount of sodium aluminate NaAlO 2 Chemical reagents, and use deionized water to prepare an aqueous solution with a concentration of 0.50 mol / L. Then weigh nickel nitrate Ni (NO 3 ) 2 ·6H 2 O and strontium nitrate Sr(NO 3 ) 2 Chemical reagents, dissolved in appropriate amount of deionized water.
[0048] (2) The sodium aluminate aqueous solution was placed in a digitally controlled temperature-adjusting ultrasonic instrument, and the nickel nitrate solution was first slowly added to it under the action of continuous stirring and ultrasonic waves at 45°C, and then the strontium nitrate solution was continued to be slowly added after 8 minutes. Then the prepared mass concentration of 1% HNO 3 The solution was slowly added dropwise into the beaker, the pH value was adjusted to 9.0, and the ultrasonic stirring was continued for 25 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com