Synthetic method of metformin hydrochloride
A technology of metformin hydrochloride and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problem of high reaction temperature, and achieve the advantages of lowering reaction temperature, simplifying purification process, reducing production cost and environmental cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Add 2.3Kg dimethylamine, 4.2Kg dicyandiamide, 5.5Kg of 30% hydrochloric acid aqueous solution successively in 20L reaction kettle with mechanical stirrer, 0.0084Kg mass fraction is 88% formic acid, stir slowly and heat to 130 ℃, Keep warm for 1.5 hours, slowly cool to room temperature;
[0028] (2) Transfer the milky white mixture obtained in step (1) to a 50L glass reactor, add 28Kg 95% ethanol, heat and reflux and stir for 30min and then cool and crystallize. Kg (yield is 93% based on dicyandiamide)
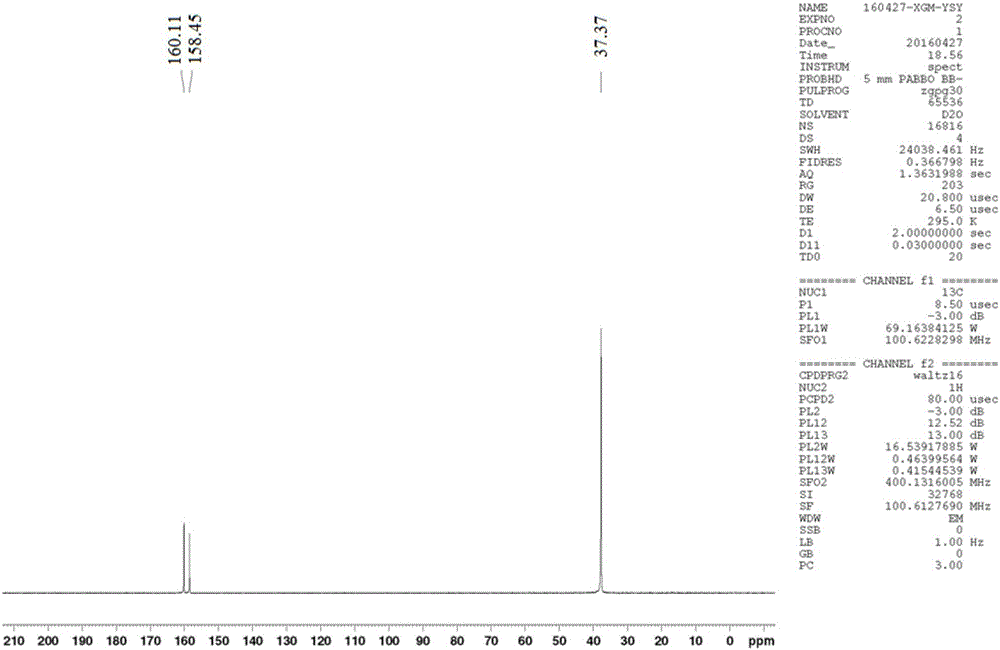
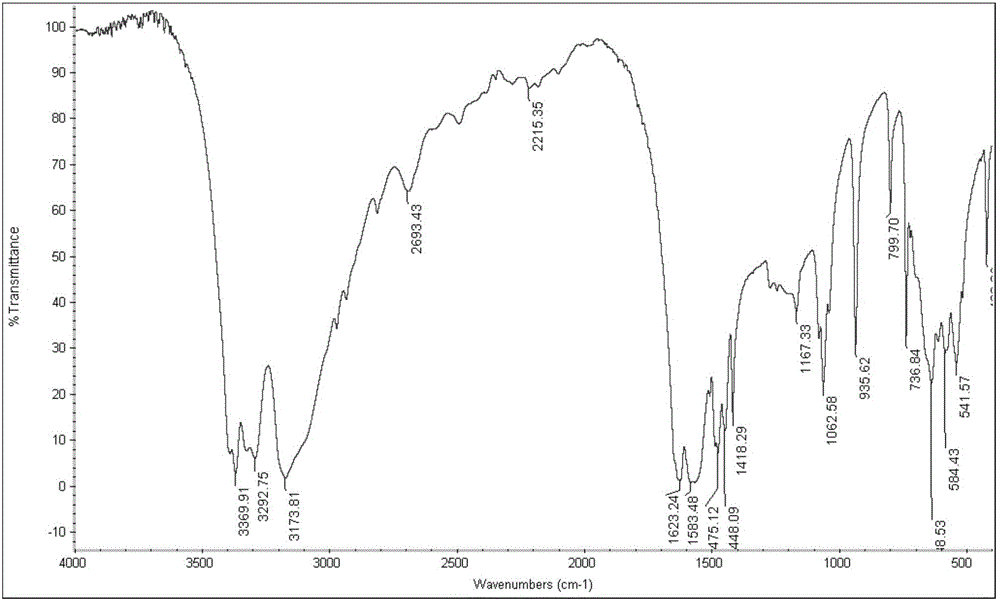
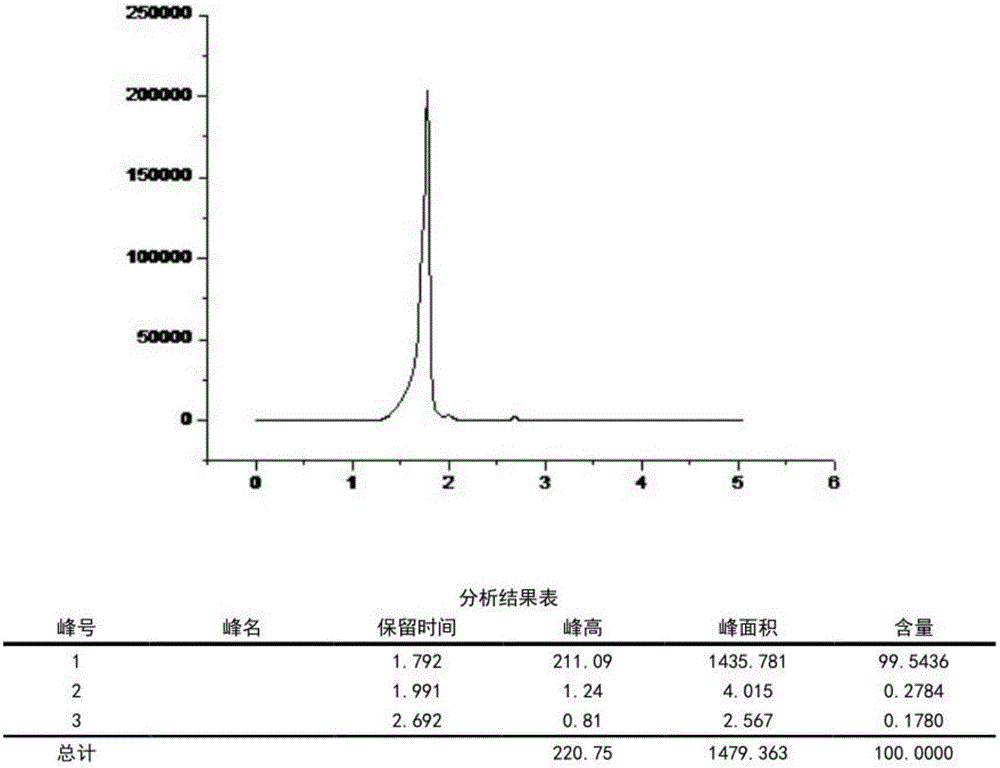
[0029] Depend on figure 1 and figure 2 It can be seen that the product prepared by the present invention is metformin hydrochloride, by image 3 It was found that the purity was higher than 95% as can be seen by high performance liquid chromatography.
Embodiment 2
[0031] (1) Add 2.3Kg dimethylamine, 4.2Kg dicyandiamide, 5.5Kg of 30% hydrochloric acid aqueous solution, 0.0084Kg mass fraction 88% formic acid successively in 20L reaction kettle with mechanical stirrer, stir slowly and heat to 120 ℃, keep warm 1.5 hours, slowly cool to room temperature;
[0032] (2) Transfer the milky white mixture obtained in step (1) to a 50L glass reactor, add 28Kg 95% ethanol, heat and reflux and stir for 30min, then cool and crystallize, after the white solid is centrifuged, vacuum-dry to obtain white metformin hydrochloride 7.45 Kg (yield is 91% based on dicyandiamide)
[0033] Depend on Figure 4 It was found that the purity was higher than 95% as can be seen by high performance liquid chromatography.
Embodiment 3
[0035] (1) Add 2.3Kg dimethylamine, 4.2Kg dicyandiamide, 8.3Kg of 20% hydrochloric acid aqueous solution, 0.0084Kg mass fraction 88% formic acid successively in a 20L reaction kettle with a mechanical stirrer, stir slowly and heat to 130°C, keep warm 1.5 hours, slowly cool to room temperature;
[0036] (2) Transfer the milky white mixture obtained in step (1) to a 50L glass reactor, add 28Kg 95% ethanol, heat and reflux and stir for 30min and then cool and crystallize. After the white solid is centrifuged, it is vacuum dried to obtain white metformin hydrochloride 7.3 Kg (yield is 88% based on dicyandiamide)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com