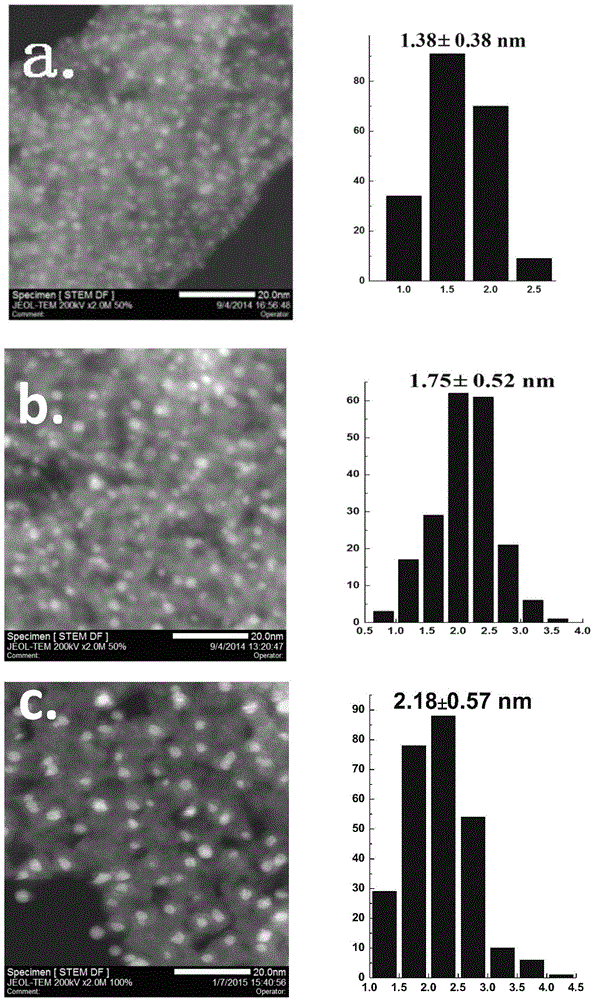
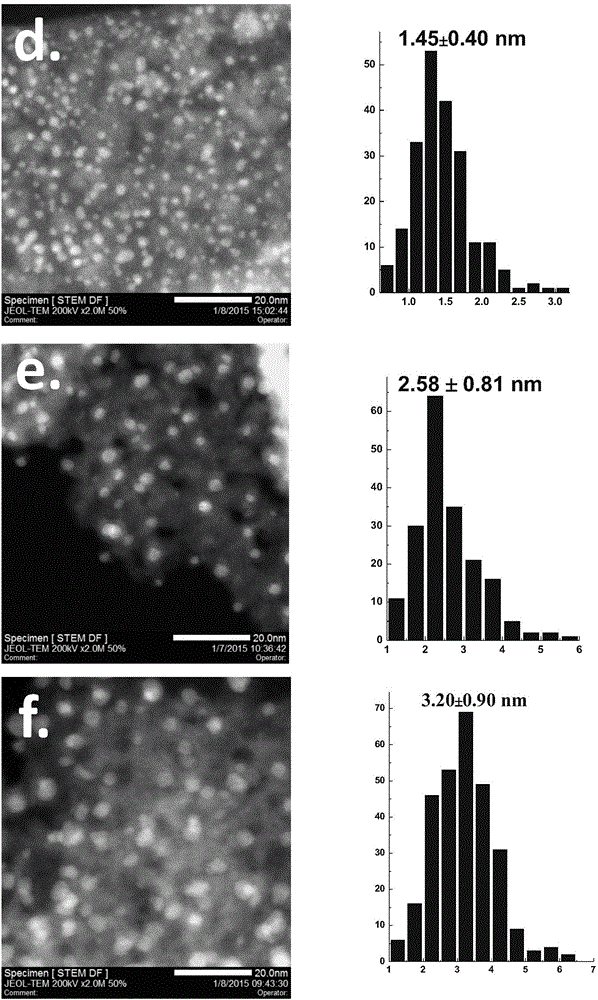
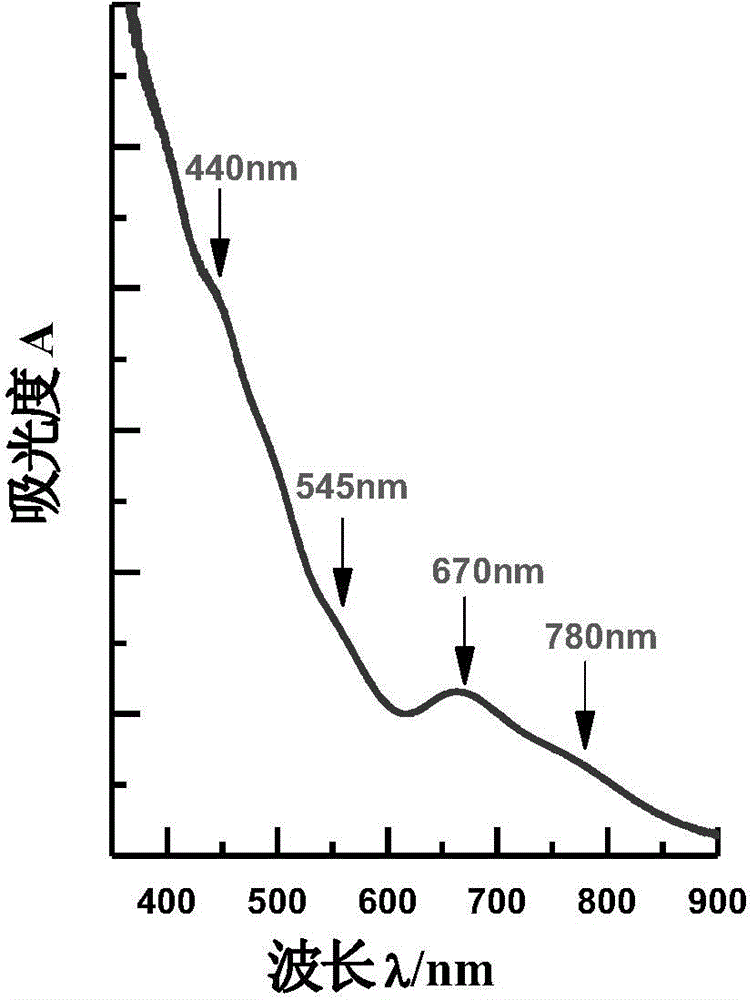
Application of supported gold cluster catalyst
A catalyst and atomic cluster technology is applied in the application field of supported gold atomic cluster catalysts to achieve the effects of mild reaction conditions, controllable particle size and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of precursors containing Au atomic clusters
[0023] Prepare chloroauric acid solution, cysteine (abbreviated as Cys) solution, 1M NaOH solution and NaBH in advance 4 solution. Wash and dry the 1000mL round bottom flask, and add magnets. Add 5.00mL HAuCl to it 4 Solution (19.12g Au / L), 200mL ultrapure and 150mL Cys solution, it was observed that the color of the solution changed from light yellow to dark yellow and finally became milky white. Then measure 30mL of 1M NaOH solution and quickly add it into the flask, and the solution immediately becomes clear and transparent. Freshly prepared NaBH was then 4 (0.2M NaOH) solution 10mL is added in the flask again, and the solution turns brownish red, and at room temperature, stirred at 1000rpm for 3h, the color of the solution gradually deepens and finally turns brownish black. In the above process, mercapto, NaOH, NaBH 4 The molar ratios to Au are 1.5, 30, 5, respectively). The obtained product was cent...
Embodiment 2
[0025] Compared with Example 1, the difference is that the cysteine solution is replaced by glutathione solution or homocysteine solution, and the amount of other materials and operating conditions are the same as in Example 1.
Embodiment 3
[0027] Preparation of hydrotalcite carrier
[0028] Take Zn(NO 3 ) 2 .6H 2 O(or Mg(NO 3 ) 2 .6H 2 O, or Ni(NO 3 ) 2 .6H 2 O, or Co(NO 3 ) 2 .6H 2 O) 0.21mol, Al(NO 3 ) 3 9H 2 Add 0.07mol of O into a 1000mL beaker, then add 200mL of deionized water and stir to dissolve to form A solution. Take NaOH 0.438mol, Na 2 CO 3 Add 0.113mol into another 500mL beaker, add 200mL deionized water and stir to dissolve to form B solution. Under stirring in a water bath at 70°C, slowly drop the A solution into the B solution with a constant flow pump, and then stir and age at this temperature for 18 hours. The resulting suspension was filtered and washed with a large amount of deionized water, and finally dried in an oven at 60°C overnight. Grind the dried sample with an agate mortar and sieve the powder with a mesh size of less than 180 mesh, bottle it and store it in a desiccator. Other hydrotalcite supports with different proportions were prepared by controlling the amount...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com