Solvent-free catalytic hydrogenation method for preparation of 2,4-dichloro-5-isopropoxy aniline
A technology of propoxyaniline and catalytic hydrogenation, which is applied in the preparation of amino compounds, the preparation of organic compounds, catalytic reactions, etc., can solve problems such as product purity decline and equipment corrosion, and achieve high product quality, low cost and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
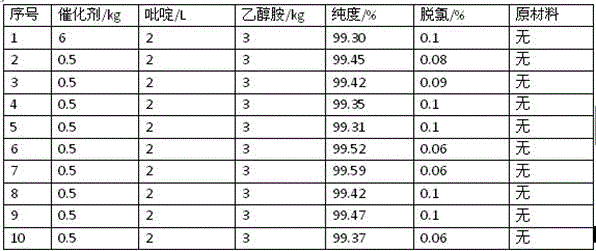
[0015] Add 1.2t of 2,4-dichloro-5-isopropoxynitrobenzene and 6kg of noble metal catalyst (self-made) into a 2m³ hydrogenation kettle, open the hydrogen valve, feed hydrogen into the hydrogenation kettle, stir and heat up the reaction , the reaction temperature is controlled at 80-100°C, the reaction pressure is controlled at 0.3-2.5MPA, the reaction speed is set at 700 rpm, and the hydrogen gas valve is closed until the hydrogen is not absorbed. The gas chromatographic analysis shows that the purity is 97.22%, the dechlorination amount is 1.9%, and the pH value of the oil phase is between 4-5.
Embodiment 2
[0017] Add 1.2t of 2,4-dichloro-5-isopropoxynitrobenzene, 6kg of noble metal catalyst (self-made), and 3kg of ethanolamine into a 2m³ hydrogenation kettle, open the hydrogen valve, and feed hydrogen into the hydrogenation kettle. Stir and heat up the reaction, the reaction temperature is controlled at 80-100°C, the reaction pressure is controlled at 0.3-2.5MPA, the reaction speed is set at 700 rpm, and the reaction is performed until no hydrogen is absorbed, the hydrogen valve is closed, the material is directly pressed, and the upper aqueous phase is separated. The purity of the crude product in the lower layer is 98.87% by gas chromatography, the dechlorination amount is 0.7%, and the pH value of the oil phase is between 5-6.
Embodiment 3
[0019] Add 1.2t of 2,4-dichloro-5-isopropoxynitrobenzene, 6kg of noble metal catalyst (self-made), and 2L of pyridine into a 2m³ hydrogenation kettle, open the hydrogen valve, and feed hydrogen into the hydrogenation kettle. Stir and heat up the reaction, the reaction temperature is controlled at 80-100°C, the reaction pressure is controlled at 0.3-2.5MPA, the reaction speed is set at 700 rpm, and the reaction is performed until no hydrogen is absorbed, the hydrogen valve is closed, the material is directly pressed, and the upper aqueous phase is separated. The purity of the crude product in the lower layer was analyzed by gas chromatography to be 98.72%, the dechlorination amount was 0.9%, and the pH value of the oil phase was between 5-6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com