Caged 2-(2-pyridyl) benzimidazole zinc complex and its preparation method and application
A technology of benzimidazole zinc and benzimidazole is applied in the field of caged 2-benzimidazole zinc complexes and the preparation thereof, and can solve the problems of low catalytic yield, long reaction time, uneven molecular weight distribution of polymers, and the like, The effects of simple preparation method, low environmental pollution and good industrial application prospect are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
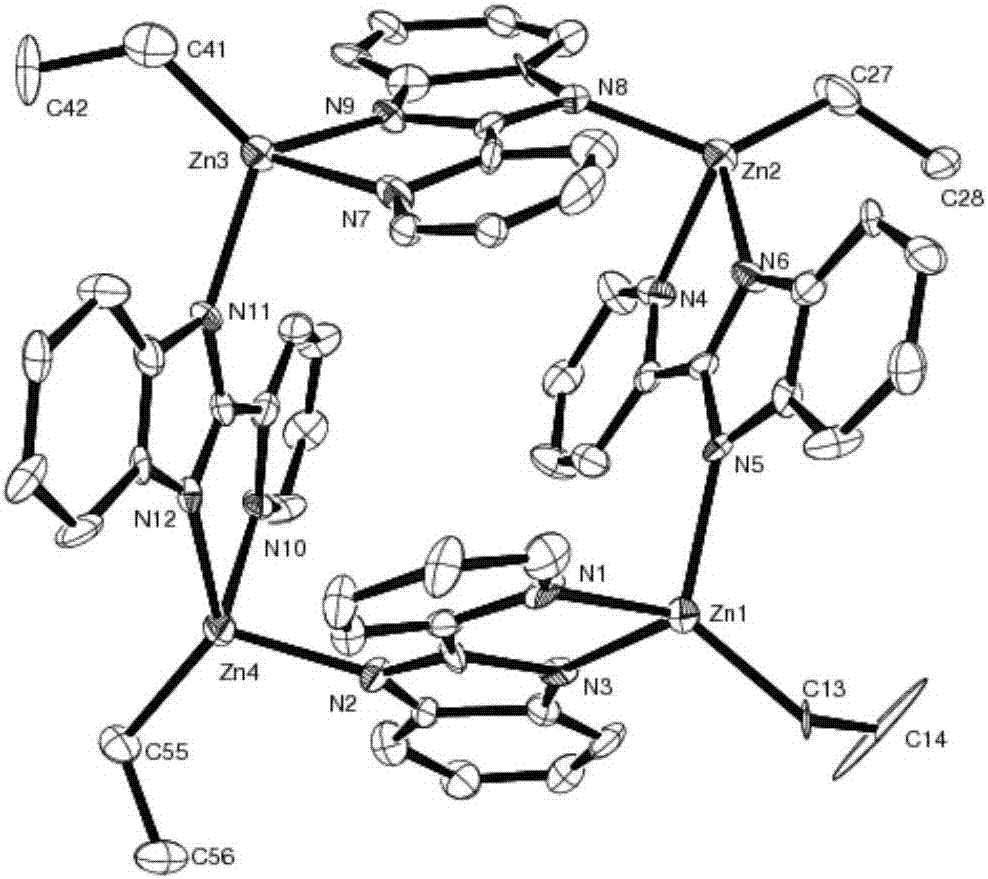
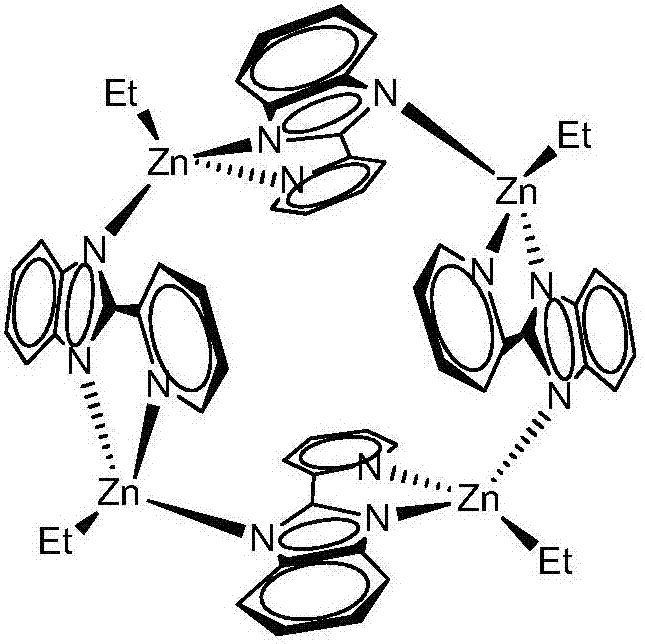
[0017] Example 1 Preparation of cage-like 2-(2-pyridyl)benzimidazole zinc complex
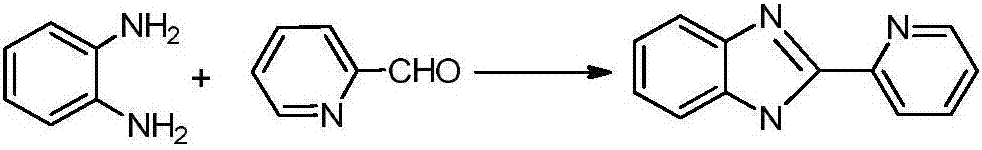
[0018] Pyridinecarboxaldehyde (2.14g, 20mmol) was slowly dropped into the ethanol solution of o-phenylenediamine (1.08g, 10mmol) at 0°C, reacted at room temperature for 4 hours, and the solvent was drained to obtain a large amount of light yellow solid. Use 5mL n-hexane respectively. Alkane was washed twice, and then recrystallized with absolute ethanol to obtain 2-(2-pyridyl)benzimidazole ligand with a yield of 94%;
[0019] Under the protection of inert gas, diethyl zinc reagent (2.0mL, 1.0M, n-hexane solution) was added dropwise to 2-(2-pyridyl)benzimidazole (2.31g, 2mmol) at -78℃. In the n-hexane solution, continue to stir for 2 hours after returning to room temperature. After the reaction is completed, filter and recrystallize. Colorless and transparent crystals are precipitated at room temperature, which is a cage-like zinc 2-(2-pyridyl)benzimidazole complex. The yield is 97%. .
[0020] Cryst...
Embodiment 2
[0023] (1) The preparation of the catalyst is the same as in Example 1
[0024] (2) Lactide ring-opening polymerization: Dissolve lactide (1.44g, 10mmol) and catalyst (0.1mmol) in 20mL of tetrahydrofuran solvent. After the solution is clarified, add benzyl alcohol (0.4mmol) for initiation, 25℃ After reacting for 1 hour, take a small amount of reaction mixture for nuclear magnetism 1 The conversion yield was detected by H NMR, and then 0.5 mL of HCl (1.0 M) ethanol solution was added to terminate the reaction, and then anhydrous ethanol was slowly added to precipitate polylactic acid. After standing, it was filtered and vacuum dried to obtain a white polylactic acid solid product. The conversion rate was 99% and PDI=1.16.
Embodiment 3
[0026] (1) The preparation of the catalyst is the same as in Example 1
[0027] (2) Lactide ring-opening polymerization: Dissolve lactide (7.20g, 50mmol) and catalyst (0.1mmol) in 30mL of tetrahydrofuran solvent, after the solution is clear, add benzyl alcohol (0.4mmol) to initiate, 25℃ After reacting for 1 hour, take a small amount of reaction mixture for NMR 1 The conversion yield was detected by H NMR, and then 0.5 mL of HCl (1.0 M) ethanol solution was added to terminate the reaction, and then anhydrous ethanol was slowly added to precipitate polylactic acid. After standing, it was filtered and vacuum dried to obtain a white polylactic acid solid product. The conversion rate was 98%, PDI=1.11.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com