Stannic oxide-loaded porous nickel oxide gas sensor material as well as preparation and application thereof
A tin oxide and sensor technology, applied in the field of material chemistry, can solve the problems of low sensitivity and poor selectivity, and achieve the effect of high adsorption area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
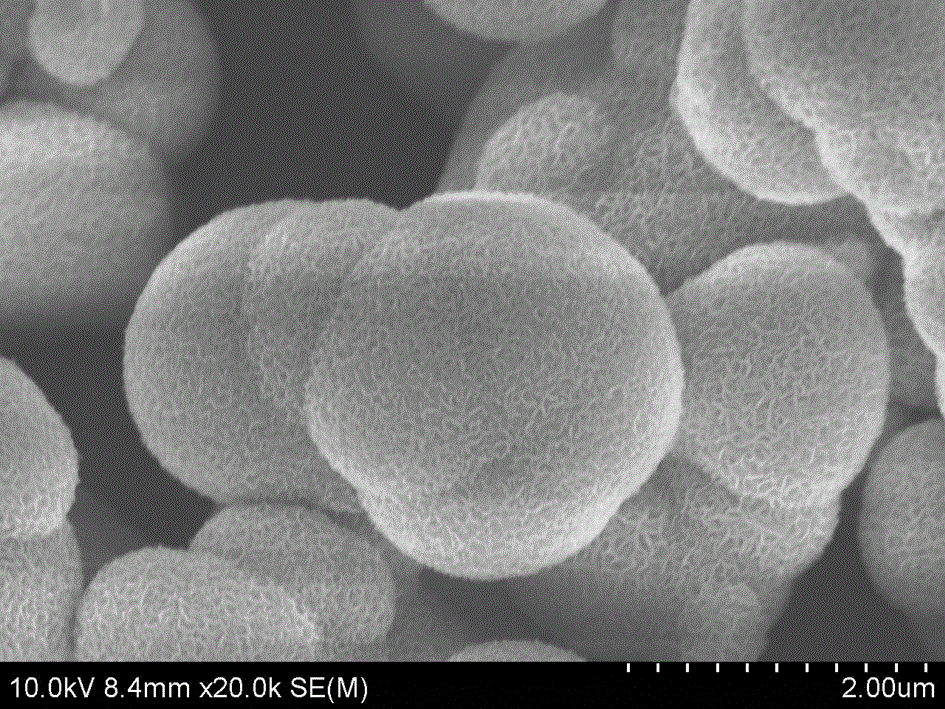
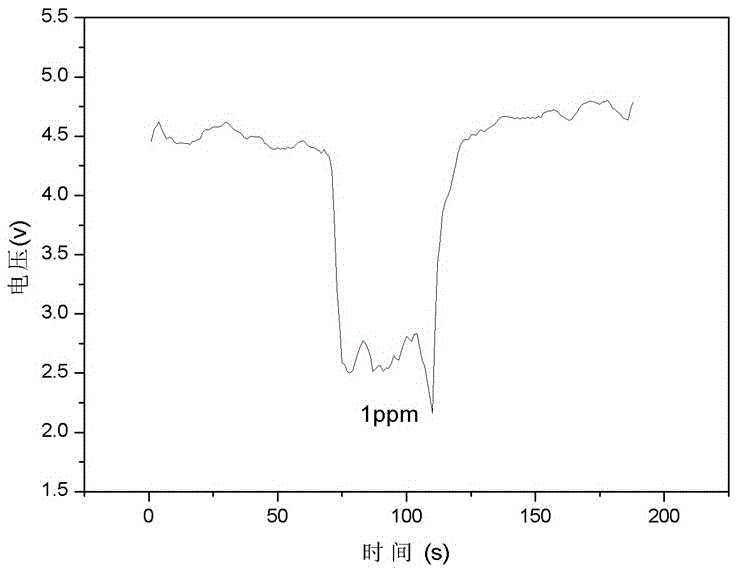
[0019] Add 2mol / L sodium hydroxide and 0.05mol / l tetrahydroxy nickel into deionized water, stir evenly, then transfer the mixed solution into a hydrothermal reaction kettle with polytetrafluoroethylene, tighten the seal, and put it in 100 o C static reaction in a constant temperature oven for 12 hours; the reaction product was repeatedly washed with deionized water until the pH value was 7~8; then washed with hydrochloric acid for 4 to 5 times; o After drying in the oven of C, porous nickel oxide spherical particles were obtained; the obtained nickel hydroxide was ultrasonically dispersed in an aqueous solution of 0.01 mol / l ammonium fluorostannate, the molar ratio of tin to nickel was 1:2, and stirred for 3 hours, Wash alternately with deionized water and ethanol at 50 o Dry in an oven at C, add n-butanol at 80 o C removes the water in the hydroxide to obtain the porous nickel oxide gas sensor material supported by tin oxide, see figure 1 ; Disperse the obtained powder on ...
Embodiment 2
[0021] Add 10mol / L sodium hydroxide and 0.2mol / lα-diimine metal nickel into deionized water, stir evenly, then transfer the mixed solution into a hydrothermal reaction kettle with polytetrafluoroethylene, tighten the seal, and put it in 160 o C static reaction in a constant temperature oven for 6 hours; the reaction product was repeatedly washed with deionized water until the pH value was 7~8; then washed with hydrochloric acid for 4 to 5 times; o After drying in the oven of C, porous nickel oxide spherical particles were obtained; the obtained nickel hydroxide was ultrasonically dispersed in 0.05 mol / l ammonium fluorostannate aqueous solution, the molar ratio of tin to nickel was 1:6, stirred for 3 hours, Wash alternately with deionized water and ethanol at 70 o Dry in an oven at C, add n-butanol, at 95 o C removes the water in the hydroxide to obtain the porous nickel oxide gas sensor material supported by tin oxide; the obtained powder is dispersed and coated on the hexapo...
Embodiment 3
[0023] Add 5mol / L sodium hydroxide and 0.1mol / l nickel sulfamate into deionized water, stir evenly, then transfer the mixed solution into a hydrothermal reaction kettle with polytetrafluoroethylene, tighten the seal, and put it in 130 o C static reaction in a constant temperature oven for 9 hours; the reaction product was repeatedly washed with deionized water until the pH value was 7~8; then washed with hydrochloric acid for 4 to 5 times; o After drying in the oven of C, porous nickel hydroxide spherical particles were obtained; the obtained nickel hydroxide was ultrasonically dispersed into an aqueous solution of 0.03 mol / l stannous oxalate, the molar ratio of tin to nickel was 1:4, and stirred for 3 hours, Wash alternately with deionized water and ethanol at 60 o Dry in an oven of C, add n-butanol, at 90 o C removes the water in the hydroxide to obtain the porous nickel oxide gas sensor material supported by tin oxide; the obtained powder is dispersed and coated on the hex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com