A Specific Probe Substrate for Cytochrome p450 2A6 Enzyme and Its Application
A technology of probe substrate and cytochrome, which is applied to the specific probe substrate of a class of cytochrome P450 2A6 enzymes and its application field, can solve the problems of inability to establish detection methods, lack of specific probes, etc., and achieve easy The effect of detection, high sensitivity, and simple source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
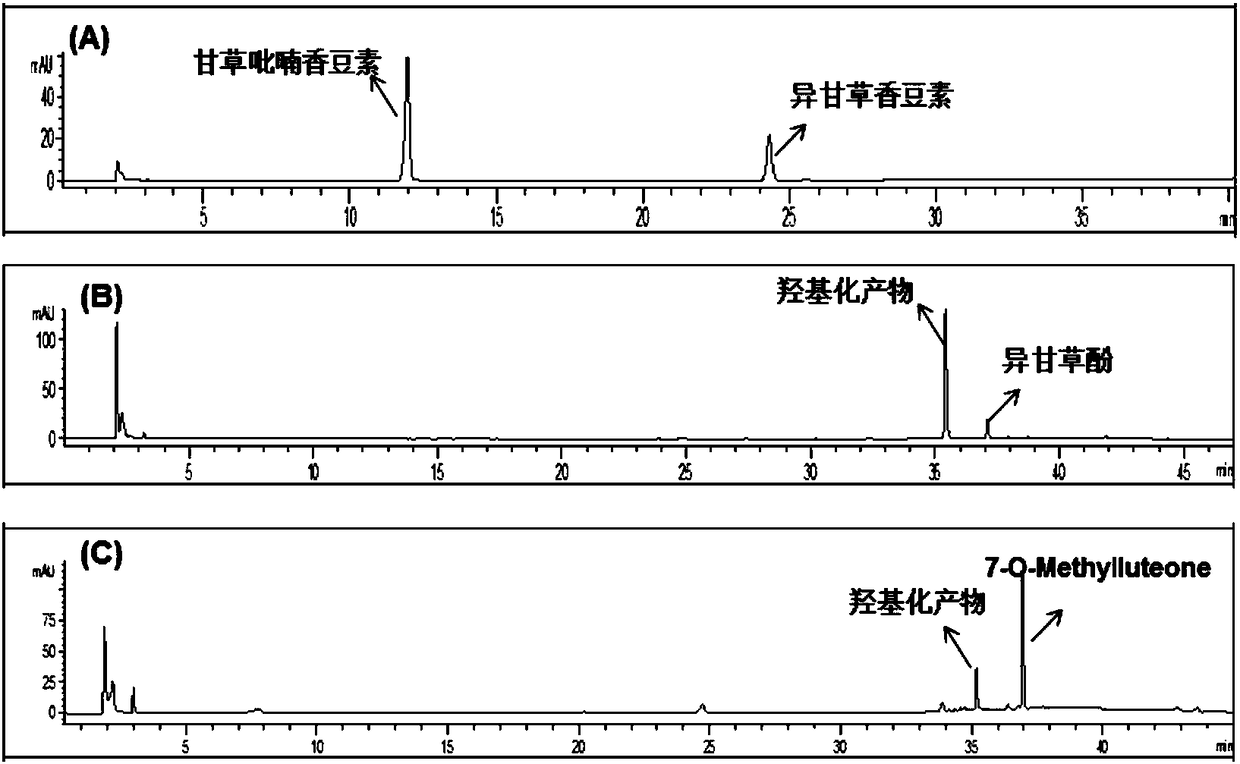
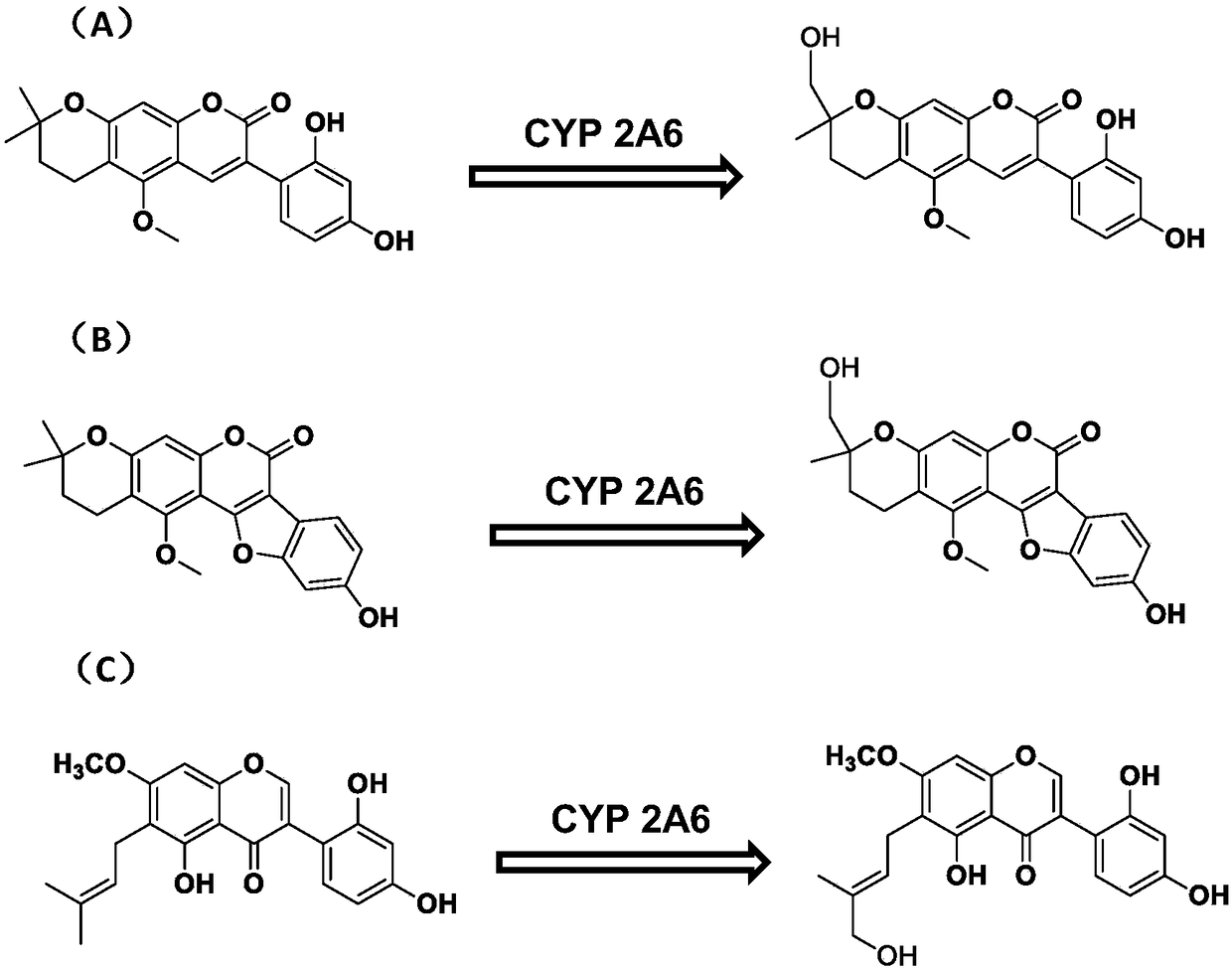
[0035] Example 1. Separation and purification of isoglycyrrhizin, licoricepyranocoumarin, isoglycyrrhizol and 7-O-methylluteone.
[0036] 1. Experimental materials and methods.
[0037] All chemical reagents mentioned in this method were purchased from Beijing Chemical Plant.
[0038] Get 35kg of licorice medicinal material (Glycyrrhiza branch of Inner Mongolia Yili Science and Technology Industrial Co., Ltd.), pulverize, extract twice with 10 times the amount of 95% ethanol and 70% ethanol, each time for 2 to 3 hours, reclaim the ethanol under reduced pressure, and the obtained The extract (10 L) was suspended in water and extracted 5 times with ethyl acetate to obtain 2500 g of the ethyl acetate fraction. Take 1280g of ethyl acetate, separate it by silica gel column chromatography (200-300 mesh, Qingdao Ocean Chemical Co., Ltd.), and elute with petroleum ether-ethyl acetate gradient (1:0-1:1) to obtain fractions A-J . Fraction I (50g) was separated by silica gel column ch...
Embodiment 2
[0041] Example 2. The use of isolicyrrhizin coumarin in the determination of 2A6 subenzyme activity in human liver microsomes.
[0042] 1. Experimental materials and methods.
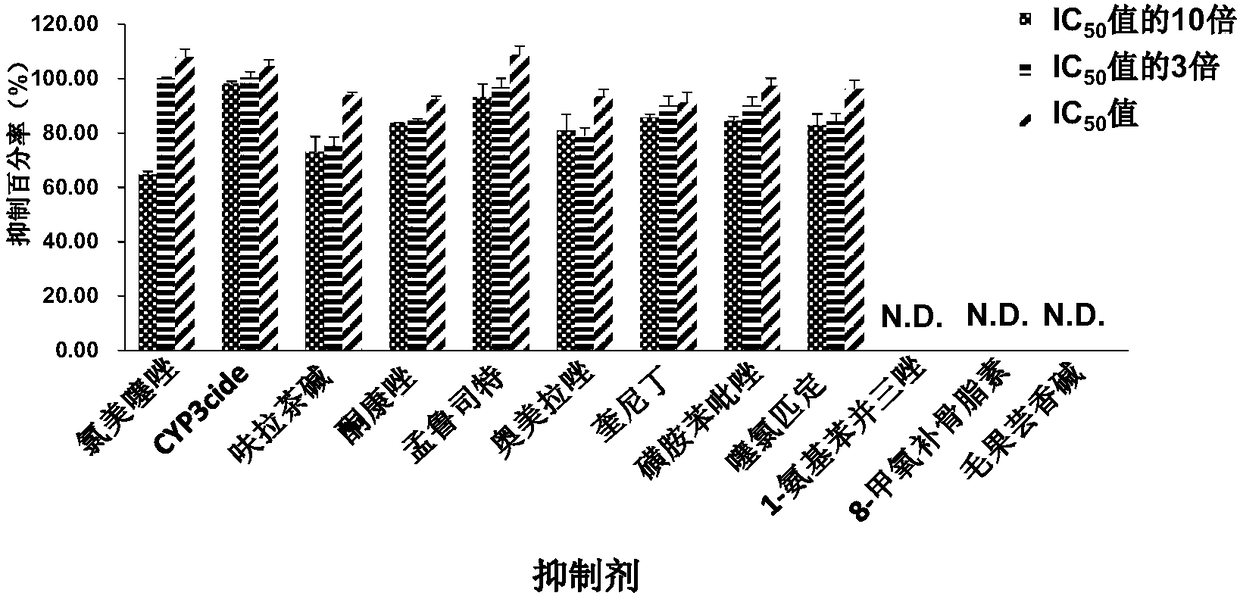
[0043] Materials: 1-aminobenzotriazole (ABT) and pilocarpine were purchased from Santa Cruz Biotechnology (Santa Cruz, California, USA) and Abcam Biochemicals (Cambridge, United Kingdom), respectively. Montelukast was purchased from Energy Chemical Ltd. (Shanghai, China). CYP3cide and clomethiazole were purchased from Toronto Research Chemical Inc. (Toronto, Ontario, Canada). Sulfafenpyrazole and furafylline were purchased from Cayman Chemical Company (Ann Arbor, Michigan, USA). 8-Methoxypsoralen, omeprazole, quinidine, ticlopidine, and ketoconazole were purchased from Sigma (St. Louis, MO, USA). Magnesium chloride and potassium phosphate were purchased from Aladdin Reagent Company. β-Nicotinamide adenine dinucleotide phosphate hydrate (β-NADP), glucose-6-phosphate (G-6-P) and glucose-6-phosphate de...
Embodiment 3
[0053] Example 3. Isoliquiritol and 7-O-Methylluteone are used for the determination of 2A6 subenzyme activity in human liver microsomes.
[0054] 1. Experimental materials and methods.
[0055] Materials: Isoglycyrrhizol and 7-O-Methylluteone were isolated from Glycyrrhiza uralensis Fisch., and the purity of the compounds reached 98% by HPLC / UV analysis. Other materials are consistent with Example 2.
[0056] Method: using the activity detection method of the probe substrate isolicyrrhizin coumarin in Example 1 after adding a selective inhibitor.
[0057] A. The concentration of selective inhibitor is IC 50 3-fold concentration of the substrate isoglycyrrhizol and 7-O-Methylluteone are both at 25 μM.
[0058] B. Add NADPH system (0.45mg / mL human liver microsomes, 2mM NADP, 8mM G-6-P, 3unit / mL G-6-P-DE, 6mM Magnesium chloride), the reaction temperature is 30-40°C, 200μL incubation system.
[0059] C. After the NADPH generating system was pre-incubated at 37°C for 30 minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com