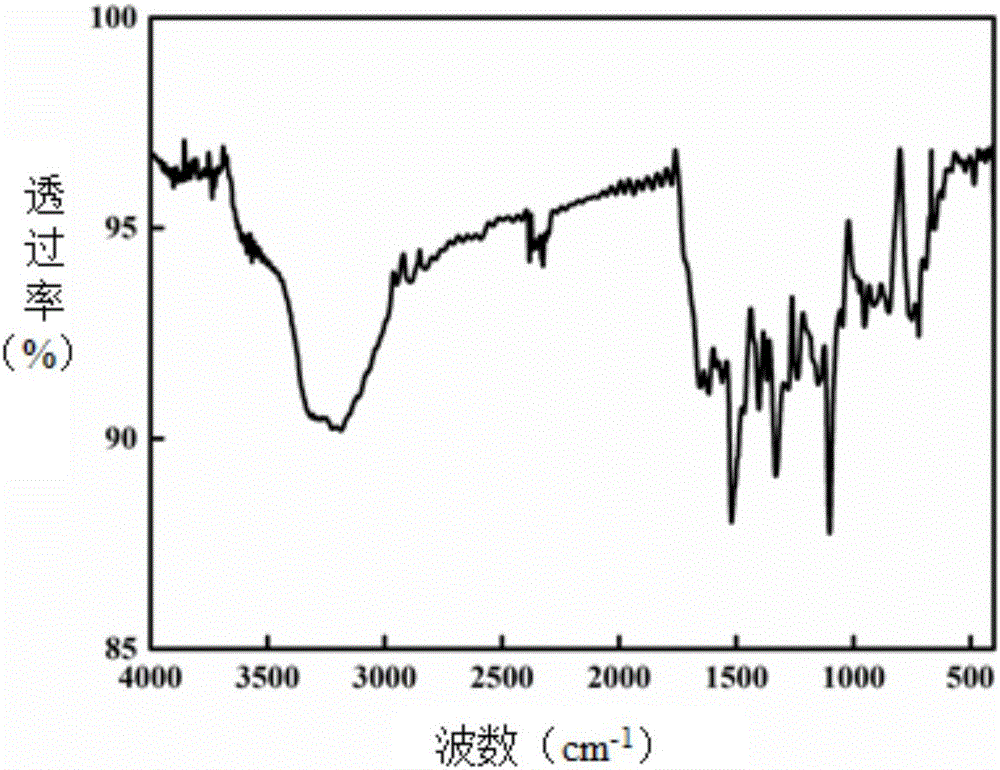
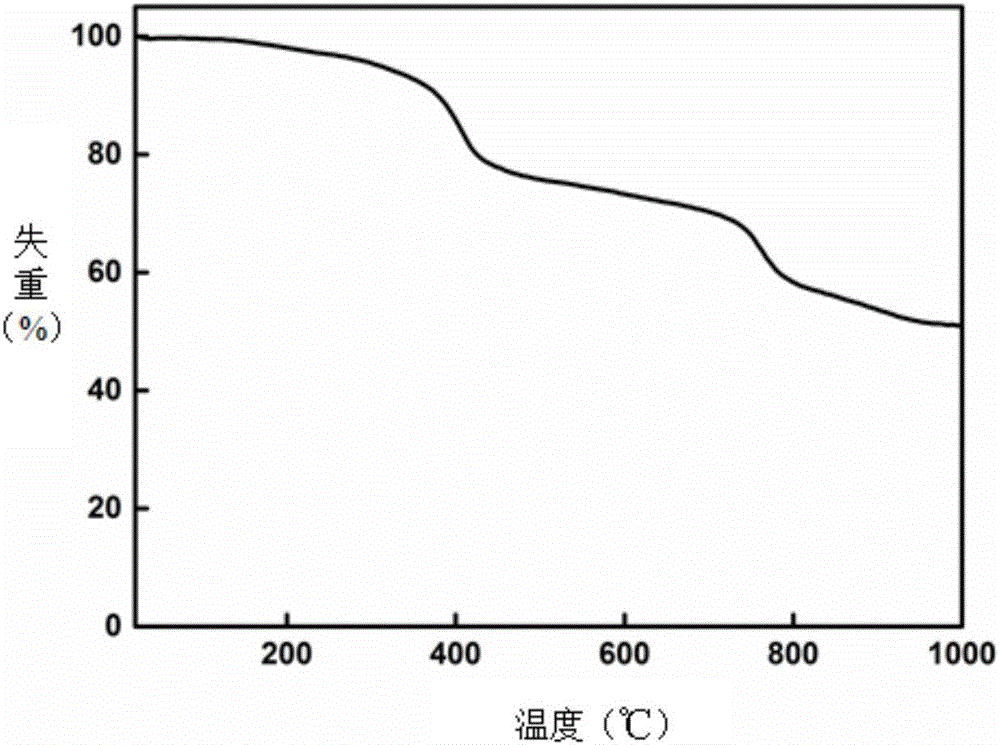
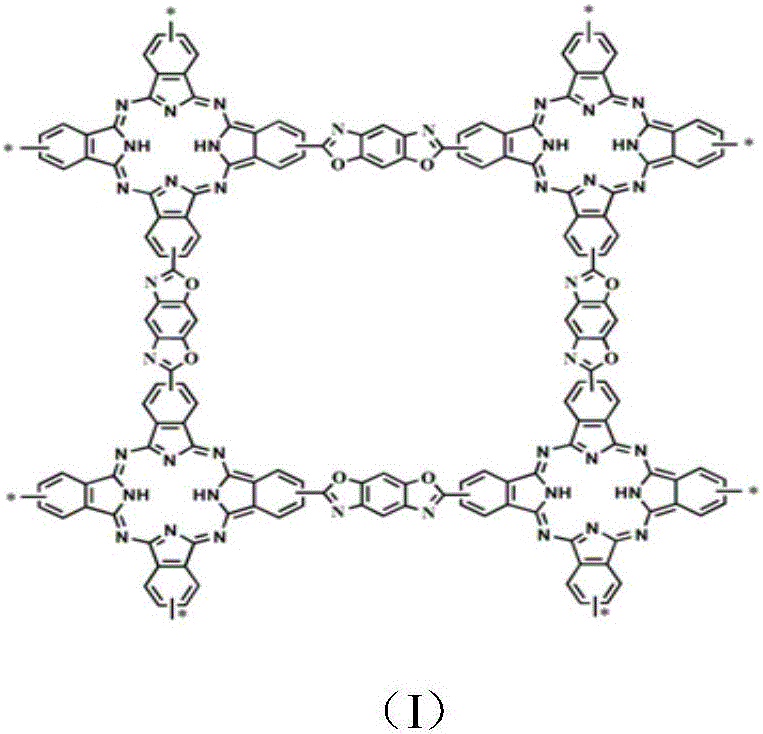
Porous reticular conjugated polymer containing phthalocyanine structural unit and preparation method of conjugated polymer
A technology of conjugated polymers and structural units, which is applied in the field of porous network conjugated polymers and its preparation, can solve problems such as easy aggregation and secondary pollution, and achieve avoidance of aggregation, improvement of stability, excellent chemical stability and The effect of heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Under nitrogen protection, in a 250mL four-neck flask, dissolve 1.71g of 4,6-diaminoresorcinol hydrochloride and 0.1mg of stannous chloride in N,N-dimethylacetamide solution, Heat and stir, slowly heat at a rate of 15°C / h, control the temperature at 30-80°C, keep each temperature for 1 hour, remove hydrogen chloride gas, and finish the reaction, weigh 3.17g of tetracarboxylic phthalocyanine in 3 times and put in In the reaction solution, the temperature was gradually raised to 155°C at a rate of 10°C / h, and each temperature was maintained for 2 hours until 155°C was maintained for 6 hours. After the reaction was completed, the reaction device was lowered to room temperature, and the solvent was distilled off under reduced pressure to obtain a solid, which was repeatedly washed with water, filtered with suction, and the crude product was vacuum freeze-dried at -20°C for 12 hours. Use Soxhlet extractor to extract the dried solid, choose N,N-dimethylformamide as the extrac...
Embodiment 2
[0037] Under nitrogen protection, in a 250mL four-necked flask, dissolve 1.71g of 4,6-diaminoresorcinol hydrochloride and 0.5mg of stannous chloride in N,N-dimethylacetamide solution, Heat and stir, slowly heat at a rate of 10°C / h, control the temperature at 30-80°C, keep each temperature for 5 hours, remove hydrogen chloride gas, and weigh 4.45g of α-tetrakis(4-carboxyphenoxy ) Phthalocyanine was put into the reaction solution five times, and the temperature was gradually raised to 155°C at a rate of 5°C / h, and each temperature was maintained for 8 hours until 155°C was maintained for 12 hours. After the reaction was completed, the reaction device was lowered to room temperature, and the solvent was distilled off under reduced pressure to obtain a solid that was repeatedly washed with water and suction filtered to obtain a solid that was vacuum freeze-dried at -40°C for 24 hours. Use Soxhlet extractor to extract the dried solid, select N,N-dimethylformamide as the extraction ...
Embodiment 3
[0039]Under nitrogen protection, in a 250mL four-necked flask, dissolve 1.71g of 4,6-diaminoresorcinol hydrochloride and 0.5mg of stannous chloride in N,N-dimethylacetamide solution, Heat and stir, heat slowly at a rate of 10°C / h, control the temperature at 30-80°C, keep each temperature for 3 hours, remove hydrogen chloride gas, and weigh 4.45g of β-tetrakis(4-carboxyphenoxy ) Phthalocyanine was put into the reaction solution three times, and the temperature was gradually raised to 155°C at a rate of 10°C / h, and each temperature was maintained for 5 hours until 155°C was maintained for 9 hours. After the reaction was completed, the solvent was distilled off under reduced pressure, and the obtained solid was repeatedly washed with water and suction filtered, and the obtained solid was vacuum freeze-dried at -40°C for 12 hours. Use Soxhlet extractor to extract the dried solid, choose N,N-dimethylformamide as the extraction solvent, control the extraction time to 24 hours, heat ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com