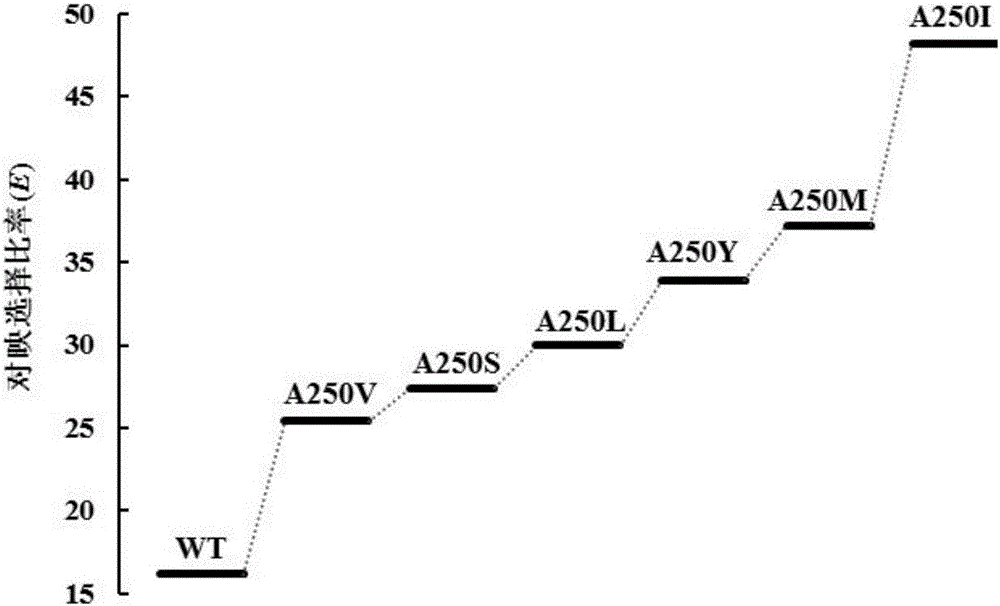
Aspergillus usamii epoxide hydrolase mutants with improved enantioselectivity
A technology of Aspergillus Usami and epoxides, applied in the field of enzyme engineering and biocatalysis, can solve the problems of low enantioselectivity and limited application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Implementation of site-specific saturation mutation
[0020] (1) Using the construction method of recombinant plasmid pET-28a(+)-Aueh2 (see the patent application of Patent Publication No. CN102994470A) as template, A250X-F and pET28-R as primers, using PrimeSTAR DNA polymerase (purchased from TaKaRa) to carry out The first round of PCR amplification (95°C 4min; 98°C 10s, 55°C 5s, 72°C 3.5min, 30 cycles; 72°C 10min) obtained a large primer A250X-1st; using the large primer A250X-1st as a primer, recombinant The plasmid pET-28a(+)-Aueh2 was used as a template for the second round of PCR amplification (95°C for 4min; 98°C for 10s, 55°C for 10s, 72°C for 3.5min, 25 cycles; 72°C for 10min); A250X-2st PCR product Transform E.coli BL21(DE3) competent cells after digesting (37°C, 2h) the template pET-28a(+)-Aueh2 with Dpn I, coat kanamycin-resistant LB plates and culture at 37°C for 12-16h to obtain Recombinant library.
[0021] A250X-F: TTTGGCAGTGGTTAC GTCGAGCA...
Embodiment 2
[0027] Example 2: Determination of Enantioselectivity of Recombinant AuEH2 and AuEH2 Mutant Enzyme
[0028]Add 500 μL of bacterial suspension and 450 μL of sodium phosphate buffer (pH 7.5) to a 1.5 mL EP tube, incubate at 10°C for 5 min, then add 50 μL of rac-SO (final concentration 10 mmol / L) for reaction. 50 μL of samples were regularly extracted to 1 mL of ethyl acetate (containing 1 mmol / L n-hexanol as an internal standard) for extraction, and the samples were analyzed using a gas chromatograph GC-2010 (Shimadzu, Japan), a chiral gas chromatography column, and a hydrogen flame ionization detector. The analysis conditions were: inlet and detector temperature 250°C; initial column temperature 100°C, rising to 195°C at 5°C / min; carrier gas nitrogen, flow rate 3.0mL / min, split ratio 1:50. The retention times of n-hexanol, (R)-styrene oxide and (S)-styrene oxide were 3.477, 5.959 and 6.065 min, respectively. Substrate e.e. s =[(S-R) / (R+S)]×100%; E=ln[(1-c)×(1-e.e. s )] / ln[(1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com