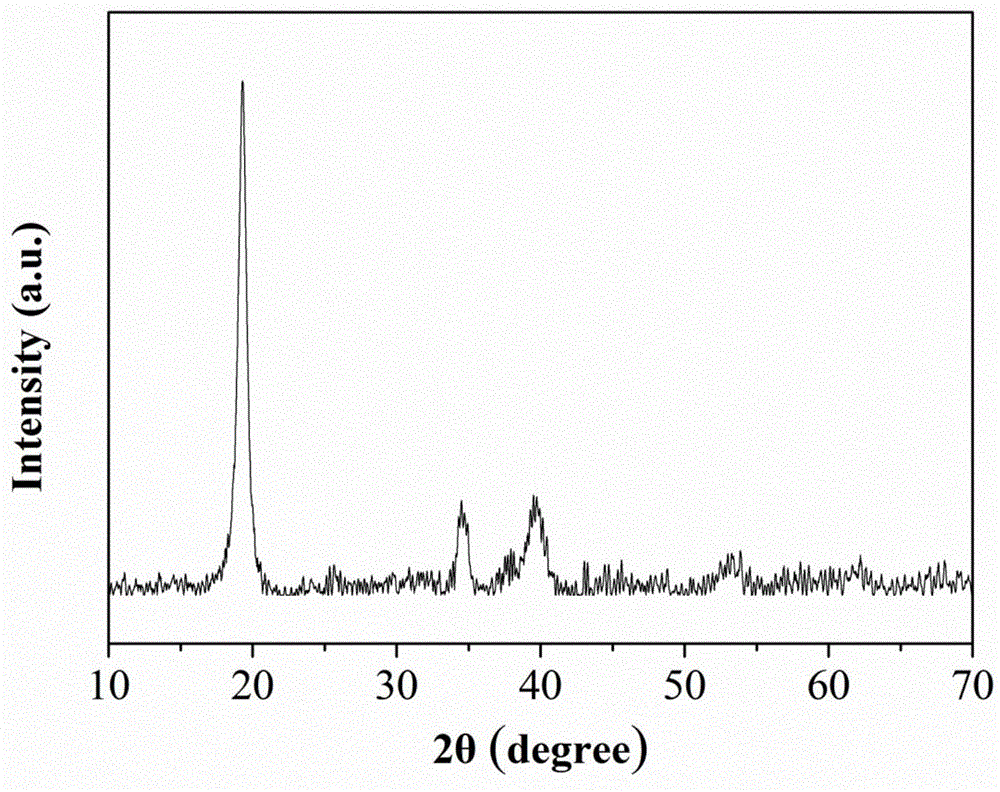
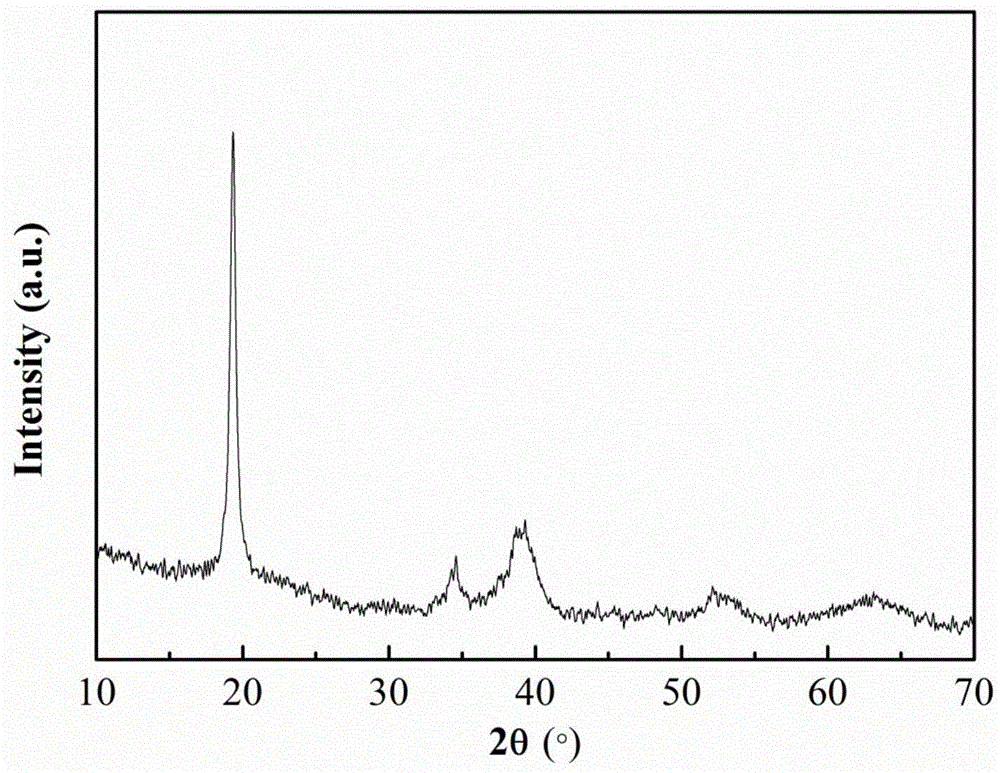
Method for continuously preparing nanoflower lithium ion battery layered anode material and reaction kettle thereof
A lithium-ion battery, cathode material technology, applied in battery electrodes, nanotechnology, nanotechnology, etc., can solve problems such as difficulty in meeting high energy density and high power density, agglomeration of precursor secondary particles, and poor rate performance of finished products. , to improve the stirring effect, strengthen the convection and diffusion, and achieve the effect of uniform distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In this embodiment, according to the chemical formula LiNi 0.33 co 0.33 mn 0.33 o 2 Batching, where x=0.33, y=0.33.
[0055] (1) Solution preparation
[0056] Accurately weigh NiSO according to the molar ratio of nickel, cobalt and manganese of 1:1:1 4 ·6H 2 O. CoSO 4 ·7H 2 O, MnSO 4 ·H 2 0 three kinds of salts, three kinds of mixtures are added in the dissolving tank, and add distilled water under normal pressure and stir until dissolving completely, be made into concentration 3mol / L mixed salt solution; L sodium hydroxide solution; Strong ammonia water is diluted to the ammonia solution of 5mol / L with deionized water;
[0057] (2) Co-precipitation
[0058] Add the mixed salt solution, sodium hydroxide solution and ammonia solution obtained in above-mentioned steps (1) respectively in the reactor, control the mixed salt solution feed flow rate to be 1mL / min, adjust the feed rate of the sodium hydroxide solution to make the reaction system pH value At 11.3±0.2...
Embodiment 2
[0064] In this embodiment, according to the chemical formula LiNi 0.5 co 0.2 mn 0.3 o 2 Batching, where x=0.5, y=0.2.
[0065] (1) Solution preparation
[0066] Accurately weigh NiCl according to the molar ratio of nickel, cobalt and manganese of 5:2:3 2 ·6H 2 O, CoCl 2 ·6H 2 O and MnCl 2 4H 2 O three kinds of salts, three kinds of mixtures are added in the dissolving tank, and add deionized water under normal pressure and stir until completely dissolving, make concentration 1mol / L mixed salt solution; Weigh sodium hydroxide solid and add deionized water to dissolve and be formulated 5mol / L sodium hydroxide solution; dilute concentrated ammonia water to 12mol / L ammonia solution with deionized water;
[0067] (2) Co-precipitation
[0068] Add the above-mentioned mixed salt solution, sodium hydroxide solution and ammonia solution to the reactor respectively, control the mixed salt solution feed flow rate to be 10mL / min, adjust the feed rate of the sodium hydroxide sol...
Embodiment 3
[0074] In this embodiment, according to the chemical formula LiNi 0.5 mn 0.5 o 2 Batching, where x=0.5, y=0.
[0075] (1) Solution preparation
[0076] Accurately weigh Ni(NO 3 ) 2 ·6H 2 O and Mn(CH 3 COO) 2 4H 2 O two kinds of raw materials, put the mixture into the dissolving tank, and add deionized water under normal pressure and stir until completely dissolved to form a mixed salt solution with a concentration of 0.5mol / L; weigh the solid sodium hydroxide and add it into deionized water to dissolve and prepare 0.5 mol / L sodium hydroxide solution; dilute concentrated ammonia water to 1mol / L ammonia solution with deionized water;
[0077] (2) Co-precipitation
[0078] Add the above-mentioned mixed salt solution, sodium hydroxide solution and ammonia solution to the reactor respectively, control the mixed salt solution feed flow rate to be 8mL / min, adjust the feed rate of the sodium hydroxide solution so that the pH value of the reaction system is at 10.3 ± 0.3, Ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com